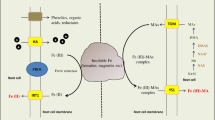
Abstract
Purpose
The rationale of this paper is to review the state of the art regarding the biotic and abiotic reactions that can influence Fe availability in soils. In soil, the management-induced change from oxic to anoxic environment results in temporal and spatial variations of redox reactions, which, in turn, affect the Fe dynamics and Fe mineral constituents. Measuring the Fe forms in organic complexes and the interaction between bacteria and Fe is a major challenge in getting a better quantitative understanding of the dynamics of Fe in complex soil ecosystems.
Materials and methods
We review the existing literature on chemical and biochemical processes in soils related with the availability of Fe that influences plant nutrition. We describe Fe acquisition by plant and bacteria, and the different Fe–organic complexes in order to understand their relationships and the role of Fe in the soil carbon cycle.
Results and discussion
Although total Fe is generally high in soil, the magnitude of its available fraction is generally very low and is governed by very low solubility of Fe oxides. Plants and microorganisms can have different strategies in order to improve Fe uptake including the release of organic molecules and metabolites able to form complexes with FeIII. Microorganisms appear to be highly competitive for Fe compared with plant roots. Crystalline Fe and poorly crystalline (hydro)oxides are also able to influence the carbon storage in soil.
Conclusion
The solubility of crystalline Fe minerals in soil is usually very low; however, the interaction with plant, microbes, and organic substance can improve the formation of soluble FeIII complexes and increase the availability of Fe for plant growth. Microbes release siderophores and plant exudates (e.g., phytosiderophores, organic acids, and flavonoids), which can bind and solubilize the Fe present in minerals. The improved understanding of this topic can enable the identification of effective solutions for remedying Fe deficiency or, alternatively, restricting the onset of its symptoms and yield’s limitations in crops. Therefore, development and testing of new analytical techniques and an integrated approach between soil biology and soil chemistry are important prerequisites.



Similar content being viewed by others
References
Adamo P, Colombo C, Violante P (1997) Occurrence of poorly ordered Fe-rich phases at the interface between the lichen Stereocaulon vesuvianum and volcanic rock from Mt Vesuvius. Clay Miner 32:453–461
Adriano AD (2001) Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals, Springer, New York Advances in Agronomy 99:183-225
Albrecht-Gary AM, Crumbliss AL (1998) Coordination chemistry of siderophores: thermodynamics and kinetics of iron chelation and release. Met Ions Biol Syst 35:239–327
Baker BJ, Banfield JF (2003) Microbial communities in acid mine drainage. FEMS Microbiol Ecol 44:139–152
Bauer I, Kappler A (2009) Rates and extent of reduction of Fe(III) compounds and O2 by humic substances. Environ Sci Technol 43:4902–4908
Borch T, Kretzschmar R, Kappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell K (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23
Borggaard OK, Jorgensen SS, Moberg JP, Raben-Lange B (1990) Influence of organic matter on phosphate adsorption by aluminium and iron oxides in sandy soils. J Soil Sci 41:443–449
Boukhalfa H, Crumbliss AL (2002) Chemical aspects of siderophore mediated iron transport. Biometals 15:325–339
Burton ED, Bush RT, Sullivan LA, Hocking RK, Mitchell DRG, Johnston SG, Fitzpatrick RW, Raven M, McClure S, Jang LY (2009) Iron-monosulfide oxidation in natural sediments: resolving microbially-mediated S transformations using XANES, electron microscopy and selective extractions. Environ Sci Technol 43:3128–3134
Burton ED, Bush RT, Johnston SG, Sullivan LA, Keene AF (2011) Sulfur biogeochemical cycling and novel Fe–S mineralization pathways in a tidally re-flooded wetland. Geochim Cosmochim Acta. doi:10.1016/j.gca.2011.03.020
Carrillo-Gonzáles R, Šimunek J, Sauvé S, Adriano D (2006) Mechanisms and pathways of trace elements mobility in soils. Adv Agron 91:112–178
Cesco S, Römheld V, Varanini Z, Pinton R (2000) Solubilization of iron by water-extractable humic substances. J Plant Nutr Soil Sci 163:285–290
Cesco S, Nikolic M, Römheld V, Varanini Z, Pinton R (2002) Uptake of 59Fe from soluble 59Fe–humate complexes by cucumber and barley plants. Plant Soil 241:121–128
Cesco S, Rombolà AD, Tagliavini M (2006) Phytosiderophores released by graminaceous species promote 59Fe uptake in citrus. Plant Soil 287:223–233
Cesco S, Neumann G, Tomasi N, Pinton R, Weisskopf L (2010) Release of plant-borne flavonoids into the rhizosphere and their role in plant nutrition. Plant Soil 329:1–25
Cesco S, Mimmo T, Tonon G, Tomasi N, Pinton R, Terzano R, Neumann G, Weisskopf L (2012) Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biol Fertil Soils 48:123–149
Cheng YQ, Yang LZ, Cao ZH, Yin S (2009) Chronosequential changes of selected pedogenic properties in paddy soils as compared with non-paddy soils. Geoderma 151:31–41
Colombo C, Torrent J (1991) Aggregation and iron oxides in Terra Rossa soils. Catena 18:51–59
Colombo C, Barrón V, Torrent J (1994) Phosphate adsorption and desorption in relation to morphology and crystal properties of synthetic hematites. Geochimica et Cosmochimica acta 58:1261–1269
Colombo C, Palumbo G, Sellitto VM, Rizzardo C, Tomasi N, Pinton R, Cesco S (2012) Characteristics of insoluble, high molecular weight iron-humic substances used as plant iron sources. Soil Sci Soc Am J 76:1246–1256
Connolly EL, Campbell N, Grotz N, Prichard CL, Guerinot ML (2003) Overexpression of the FRO2 iron reductase confers tolerance to growth on low iron and uncovers post-transcriptional control. Plant Physiol 133:1102–1110
Cornell RM, Schwertmann U (2003) The iron oxides, 2nd edn. Wiley-VCH, Weinheim
Cornell RM, Giovanoli R, Schneider W (1989) Review of the hydrolysis of iron (III) and the crystallization of amorphous iron (III) hydroxide hydrate. J Chem Technol Biotechnol 46:115–134
de Santiago A, Delgado A (2006) Predicting iron chlorosis of Lupinus albus L. in calcareous Spanish soils from various iron extracts. Soil Sci Soc Am J 70:1945–1950
Dehner CA, Awaya JD, Maurice PA, DuBois JL (2010) Roles of siderophores, oxalate, and ascorbate in mobilization of iron from hematite by the aerobic bacterium Pseudomonas mendocina. Appl Environ Microbiol 76:2041–2048
Dhungana S, Anthony CR III, Hersman LE (2007) Effect of exogenous reductant on growth and iron mobilization from ferrihydrite by the Pseudomonas mendocina ymp strain. Appl Environ Microbiol 73:3428–3430
Duckworth OW, Holmstrom SJM, Pena J, Sposito G (2009) Biogeochemistry of iron oxidation in a circumneutral freshwater habitat. Chem Geol 260:149–158
Duijff BJ, Bakker PAHM, Schippers B (1994) Ferric pseudobactin 358 as an iron source for carnation. J Plant Nutr 17:2069–2078
El Hajji H, Nkhili E, Tomao V, Dangles O (2006) Interactions of quercitin with iron and copper ions: complexation and autoxidation. Free Radic Res 40:303–320
Fiedler S, Vepraskas MJ, Richardson JL (2007) Soil redox potential: importance, field measurements, and observations. Adv Agron 94:1–57
Fortin D, Langley S (2005) Formation and occurrence of biogenic iron-rich minerals. Earth-Sci Rev 72:1–19
Guerinot ML (1994) Microbial iron transport. Annu Rev Microbiol 48:743–772
Guerinot ML, Yi Y (1994) Iron: nutritious, noxious, and not readily available. Plant Physiol 104:815–820
Hansel CM, Benner SG, Nico P, Fendorf S (2004) Structural constraints of ferric (hydr)oxides on dissimilatory iron reduction and the fate of Fe(II). Geochim Cosmochim Acta 68:3217–3229
Heitmann T, Goldhammer T, Beer J, Blodau C (2007) Electron transfer of dissolved organic matter and its potential significance for anaerobic respiration in a northern bog. Glob Chang Biol 13(8):1771–1785
Hinsinger P (1998) How do plant roots acquire mineral nutrients? Chemical processes involved in the rhizosphere. Adv Agron 64:225–265
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant Soil 248:43–59
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M et al (2006) Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 45:335–346
Jakobsen R, Postma D (1999) Redox zoning, rates of sulfate reduction and interactions with Fe-reduction and methanogenesis in a shallow sandy aquifer, Romo, Denmark. Geochim Cosmochim Acta 63:137–151
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Jones D, Wilson MJ (1985) Chemical activity of lichens on mineral surfaces. Int Biodeterior 21:99–105
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152
Kögel-Knabner I, Guggenberger G, Kleber M, Kandeler E, Kalbitz K, Scheu S, Eusterhues K, Leinweber P (2008) Organo-mineral associations in temperate soils: integrating biology, mineralogy, and organic matter chemistry. J Plant Nutr Soil Sci 171:61–82
Kögel-Knabner I, Amelung W, Cao X, Fiedler S, Frenzel P, Jahn R, Kalbitz K, Kölbl A, Schloter M (2010) Biogeochemistry of paddy soils. Geoderma 157:1–14
Kostka JE, Stucki JW, Nealson KH, Wu J (1996) Reduction of structural Fe(III) in smectite by a pure culture of Shewanella putrefaciens strain MR-1. Clay Clay Miner 44:522–529
Kraemer SM (2004) Iron oxide dissolution and solubility in the presence of siderophores. Aquat Sci 66:3–18
Lalonde K, Mucci A, Ouellet A, Gélinas Y (2012) Preservation of organic matter in sediments promoted by iron. Nature 483:198–200
Lemanceau P, Expert D, Gaymard F, Bakker PAHM, Briat JF (2009) Role of iron in plant–microbe interactions. Adv Bot Res 51:491–549
Lindsay WL (1979) Chemical equilibria in soils. Wiley-Interscience, New York
Lindsay WL (1988) Solubility and redox equilibria of iron compounds in soils. In: Stucki JW (ed) Iron in soils and clay minerals. Reidel Publ. NATO, Dordrecht, pp 37–60
Lindsay WL, Schwab AP (1982) The chemistry of iron in soils and its availability to plants. J Plant Nutr 5:821–840
Liu F, He JZ, Colombo C, Violante A (1999) Competition in adsorption of sulfate and oxalate on goethite in absence and presence of phosphate. Soil Sci 1643:180–189
Liu F, Colombo C, Adamo P, He JZ, Violante A (2002) Manganese–iron nodules minerals associated with trace elements in Alfisols from China. Soil Sci Soc Am J 66:661–670
Liu W, Xu X, Wu X, Yang Q, Luo Y, Christie P (2006) Decomposition of silicate minerals by Bacillus mucilaginosus I liquid culture. Environ Geochem Health 28:133–140
Loeppert RH, Hossner LR, Chmielewski MA (1984) Indigenous soil properties influencing the availability of Fe in calcareous hot spots. J Plant Nutr 7:135–147
Loper JE, Buyer JS (1991) Siderophores in microbial interactions on plant surfaces. Mol Plant Microbe Interact 4:5–13
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287
Lovley DR, Phillips EJP (1986) Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol 51:683–689
Lovley DR, Phillips EJP (1988) Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron and manganese. Appl Environ Microbiol 54:1472–1480
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49:219–286
Lowenstam HA (1981) Minerals formed by organisms. Science 211:1126–1131
Lower SK, Hochella MF, Beveridge TJ (2001) Bacterial recognition of mineral surfaces: nanoscale interactions between Shewanella and α-FeOOH. Science 292:1360–1363
Lucena JJ (2000) Effect of bicarbonate, nitrate and other environmental factors on iron deficiency chlorosis. A review. J Plant Nutr 23:1591–1606
Ma JF, Shinada T, Matsuda C, Nomoto K (1995) Biosynthesis of phyto-siderophores, mugineic acids, associated with methionine cycling. J Biol Chem 270:16549–16554
Marschner P, Crowley DE (1998) Phytosiderophore decrease iron stress and pyo-verdine production of Pseudomonas fluorescens Pf-5 (pvd-inaZ). Soil Biol Biochem 30:1275–e1280
Marschner H, Römheld V (1994) Strategies of plants for acquisition of iron. Plant Soil 165:261–274
Mengel K (1994) Iron availability in plants tissues-iron chlorosis on calcareous soils. Plant Soil 165:275–283
Mengel K, Kirkby E, Kosegarten H, Appel T (2001) Iron. In: Mengel K, Kirkby EA (eds) Mineral nutrition, 5th edn. Kluwer Academic Publishers, Dordrecht, pp 553–571
Munch JC, Ottow JCG (1980) Preferential reduction of amorphous to crystalline iron oxides by bacterial activity. J Soil Sci 129:15–21
Nagasaka S, Takahashi M, Nakanishi-Itai R, Bashir K, Nakanishi H, Mori S, Nishizawa NK (2009) Time-course analysis of gene expression over 24 hours in Fe-deficient barley roots. Plant Mol Biol 69:621–631
Neilands JB (1981) Iron absorption and transport in microorganisms. Annu Rev Nutr 1:27–46
Pinton R, Cesco S, De Nobili M, Santi S, Varanini Z (1997a) Water- and pyrophosphate-extractable humic substances fractions as a source of iron for Fe-deficient cucumber plants. Biol Fertil Soils 26:23–27
Pinton R, Cesco S, Santi S, Varanini Z (1997b) Soil humic substances stimulate proton release by intact oat seedling roots. J Plant Nutr 20:857–869
Pinton R, Cesco S, Santi S (1999) Water-extractable humic substances enhance iron deficiency responses by Fe-deficient cucumber plants. Plant Soil 210:145–157
Raaijmakers J, Van de Sluis L, Koster M, Bakker PAHM, Weisbeek PJ, Schippers B (1995) Utilisation of heterologous siderophores and rhizosphere competence of fluorescent Pseudomonas spp. Can J Microbiol 41:126–135
Robin A, Vansuyt G, Hinsinger P, Meyer JM, Briat JF, Lemanceau P (2008) Iron dynamics in the rhizosphere: consequences for plant health and nutrition. Adv Agron 99:83–225
Roden EE, Zachara JM (1996) Microbial reduction of crystalline iron(III) oxides: influence of oxides surface area and potential for cell growth. Environ Sci Technol 30:1618–1628
Römheld V, Marschner H (1986) Mobilization of iron in rhizosphere of different plant species. In: Tinker PBH, Laüchli A (eds) Advances in plant nutrition, Volume 2. pp 155–204
Schnitzer M (1978) Humic substances: chemistry and reactions. In: Schnitzer M, Khan SU (eds) Soil organic matter. Elsevier, New York, pp 1–6
Schwertmann U (1985) The effect of pedogenic environments on iron oxide minerals. Adv Soil Sci 1:172–200
Schwertmann U (1988) Occurrence and formation of iron oxides in various pedoenvironments. In: Stucki JW, Goodman BA, Schwertmann U (eds) Iron in Soils, Clay Minerals. D. Reidel, Dordrecht, pp 267–308, \1
Schwertmann U (1990) Solubility and dissolution of iron oxides. In: Chen Y, Hadar Y (eds) Iron nutrition and interactions in plants. Kluwer Acad. Pub, Boston
Schwertmann U (1991) Solubility and dissolution of iron oxides. Plant Soil 130:1–25
Shaw LJ, Morris P, Hooker JE (2006) Perception and modification of plant flavonoid signals by rhizosphere microorganisms. Environ Microbiol 8:1867–1880
Sobolev D, Roden EE (2004) Characterization of a neutrophilic, chemolithoautotrophic Fe(II)-oxidizing beta-proteobacterium from freshwater wetland sediments. Geomicrobiol J 21:1–10
Sposito G (1989) The chemistry of soils. Oxford University Press, New York, 277 pp
Stackebrandt E, Sproer C, Rainey FA, Burghardt J, Pauker O, Hippe H (1997) Phylogenetic analysis of the genus Desulfotomaculum: evidence for the misclassification of Desulfotomaculum guttoideum and description of Desulfotomaculum orientis as Desulfosporosinus orientis gen. nov., comb. nov. Int J Syst Bacteriol 47:1134–1139
Stevenson FJ (1994) Humus chemistry: genesis, composition, reactions, 2nd edn. Wiley, New York, p 496
Stubner S (2002) Enumeration of 16S rDNA of Desulfotomaculum lineage 1 in rice field soil by real-time PCR with SybrGreen detection. J Microbiol Methods 50:155–164
Stucki JW, Lee K, Zhang L, Larson RA (2002) Effects of iron oxidation state on the surface and structural properties of smectites. Pure Appl Chem 74:2145–2158
Stumm W, Furrer G (1987) The dissolution of oxides and aluminium silicates: examples of surface-coordination-controlled kinetics. In: Stumm W (ed) Aquatic Surface Chemistry. John Wiley and Sons, New York, pp 197–219
Tabak HH, Lens P, van Hullebusch ED, Dejonghe W (2005) Developments in bioremediation of soils and sediments polluted with metals and radionuclides 1. Microbial processes and mechanisms affecting bioremediation of metal contamination and influencing metal toxicity and transport. Rev Environ Sci Bio/Technol 4:115–156
Takahashi Y, Minai Y, Ambe S, Makide Y, Ambe F, Tominaga T (1997) Simultaneous determination of stability constants of humate complexes with various metal ions using multitracer technique. Sci Total Environ 198:61–71
Tomasi N, Weisskopf L, Renella G, Landi L, Pinton R, Varanini Z, Nannipieri P, Torrent J, Martinoia E, Cesco S (2008) Flavonoids of white lupin roots participate in phosphorus mobilization from soil. Soil Biol Biochem 40:1971–1974
Tomasi N, Kretzschmar T, Espen L, Weisskopf L, Fuglsang AT, Palmgren MG, Neumann G, Varanini Z, Pinton R, Martinoia E, Cesco S (2009a) Plasma membrane H-ATPase-dependent citrate exudation from cluster roots of phosphate-deficient white lupin. Plant Cell Environ 32:465–475
Tomasi N, Rizzardo C, Monte R, Gottardi S, Jelali N, Terzano R, Vekemans B, De Nobili M, Varanini Z, Pinton R, Cesco S (2009b) Micro-analytical, physiological and molecular aspects of Fe acquisition in leaves of Fe-deficient tomato plants re-supplied with natural Fe-complexes in nutrient solution. Plant Soil 325:25–38
Torrent J, Cabedo A (1986) Sources of iron oxides in reddish brown soil profiles from calcarenites in Southern Spain. Geoderma 37:57–66
Treeby M, Marschner H, Romheld V (1989) Mobilization of iron and other micronutrient cations from calcareous soil by plant-borne, microbial, and synthetic metal chelators. Plant Soil 114:217–226
van Hees PAW, Lundstrom US (2000) Equilibrium models of aluminium and iron complexation with different organic acids in soil solution. Geoderma 94:201–221
Vansuyt G, Robin A, Briat JF, Curie C, Lemanceau P (2007) Iron acquisition from Fe-pyoverdine by Arabidopsis thaliana. Mol Plant Microbe Interact 20:441–447
Von Wiren N, Roemheld V, Morel JL, Guckert A, Marschner H (1993) Influence of microorganisms on iron acquisition in maize. Soil Biol Biochem 25:371–376
Von Wiren N, Mori S, Marschner H, Römheld V (1994) Iron inefficiency in maize mutant ys1 (Zea mays L. cv Yellow-Stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiol 106:71–77
Weiss JV, Emerson D, Megonigal JP (2004) Geochemical control of microbial Fe(III) reduction potential in wetlands: comparison of the rhizosphere to non-rhizosphere soil. FEMS Microbiol Ecol 48:89–100
Weiss JV, Emerson D, Megonigal JP (2005) Rhizosphere iron(III) deposition and reduction in a Juncus effusus L.-dominated wetland. Soil Sci Soc Am J 69:1861–1870
Welch SA, Barker WW, Banfield J (1999) Microbial extracellular polysacchrides and plagioclase dissolution. Geochim Cosmochim Acta 63:1405–1419
Wiseman CLS, Püttmann W (2005) Soil organic carbon and its sorptive preservation in central Germany. Eur J Soil Sci 56:65–76
Xiong H, Kakei Y, Kobayashi T, Guo X, Nakazono M, Takahashi H, Nakanishi H, Shen H, Zhanh F, Nishizawa NK, Zuo Y (2013) Molecular evidence for phytosiderophore-induced improvement of iron nutrition of peanut intercropped with maize in calcareous soil. Plant Cell Environ. doi:10.1111/pce.12097
Yu T, Bishop PL (2001) Stratification and oxidation-reduction potential change in an aerobic and sulfate-reducing biofilm studied using microelectrodes. Water Environ Res 73:368–373
Acknowledgments
Colombo C. would like to thank the financial support from the CAS as Visiting Professor Programs 2011–2012 to the Research Centre for Eco-environmental Sciences of Beijing. This research was supported by grants from Italian MIUR (FIRB-Programma “Futuro in Ricerca” and PRIN), Free University of Bolzano (TN5056), and the Natural Science Foundation of China (51221892, 41090281). The authors would like to thank Dr. Rebeka Fijan and Dr. Tanja Mimmo (Free University of Bolzano, Italy) and Tom Thomas W. Crawford, Jr., Ph.D. (Director of Research Bio Huma Netics Inc.) for their critical revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor Hailong Wang
Rights and permissions
About this article
Cite this article
Colombo, C., Palumbo, G., He, JZ. et al. Review on iron availability in soil: interaction of Fe minerals, plants, and microbes. J Soils Sediments 14, 538–548 (2014). https://doi.org/10.1007/s11368-013-0814-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0814-z