Abstract
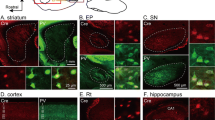
Although the functional neuroanatomy of the midbrain dopamine (mDA) system has been well characterized, the literature regarding its capacity to innervate the hippocampal formation has been inconsistent. The lack of expression of definitive markers for dopaminergic fibers, such as the dopamine transporter, in the hippocampus has complicated studies in this area. Here we have used immunohistochemical techniques to characterize the tyrosine hydroxylase expressing fiber network in the rat hippocampus, combined with retrograde tracing from the dentate gyrus to assess the capacity for afferent innervation by mDA neurons. The results indicate that virtually all tyrosine hydroxylase fibers throughout the hippocampus are of a noradrenergic phenotype, while the overlying cortex contains both dopaminergic and noradrenergic fiber networks. Furthermore, retrograde tracing from the dentate gyrus robustly labels tyrosine hydroxylase-immunoreactive noradrenergic neurons in the locus coeruleus but not mDA neurons.
Similar content being viewed by others
References
Amaral D G, Cowan W M (1980). Subcortical afferents to the hippocampal formation in the monkey. J Comp Neurol, 189(4): 573–591
Baker S A, Baker K A, Hagg T (2004). Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci, 20(2): 575–579
Ben Abdallah NM, Slomianka L, Vyssotski A L, Lipp H P (2010). Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging, 31(1): 151–161
Bischoff S, Scatton B, Korf J (1979). Biochemical evidence for a transmitter role of dopamine in the rat hippocampus. Brain Res, 165 (1): 161–165
Bjorklund A (1978). Monoaminergic inputs to the hippocampus. In: Symposium, C.F. (ed), Functions of the Septo-Hippocampal System. Elsevier Excerpta Medica North-Holland, Amsterdam
Björklund A, Dunnett S B (2007). Dopamine neuron systems in the brain: an update. Trends Neurosci, 30(5): 194–202
Borgkvist A, Malmlöf T, Feltmann K, Lindskog M, Schilström B (2012). Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int J Neuropsychopharmacol, 15(4): 531–540
Broussard J I, Yang K, Levine AT, Tsetsenis T, Jenson D, Cao F, Garcia I, Arenkiel B R, Zhou F M, de Biasi M, Dani J A (2016). Dopamine regulates aversive contextual learning and associated in vivo synaptic plasticity in the hippocampus. Cell Reports, 14(8): 1930–1939
Carr D B, Sesack S R (2000). GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse, 38 (2): 114–123
Creed M C, Ntamati N R, Tan K R (2014). VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front Behav Neurosci, 8: 8
Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza P V, Abrous D N (2003). Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci USA, 100(24): 14385–14390
Dubois A, Savasta M, Curet O, Scatton B (1986). Autoradiographic distribution of the D1 agonist [3H]SKF 38393, in the rat brain and spinal cord. Comparison with the distribution of D2 dopamine receptors. Neuroscience, 19(1): 125–137
Emre M (2003). Dementia associated with Parkinson’s disease. Lancet Neurol, 2(4): 229–237
Freundlieb N, François C, Tandé D, Oertel W H, Hirsch E C, Höglinger G U (2006). Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci, 26(8): 2321–2325
Gasbarri A, Sulli A, Packard M G (1997). The dopaminergic mesencephalic projections to the hippocampal formation in the rat. Prog Neuropsychopharmacol Biol Psychiatry, 21(1): 1–22
Gasbarri A, Verney C, Innocenzi R, Campana E, Pacitti C (1994). Mesolimbic dopaminergic neurons innervating the hippocampal formation in the rat: a combined retrograde tracing and immunohistochemical study. Brain Res, 668(1-2): 71–79
Harley C W (2007). Norepinephrine and the dentate gyrus. Prog Brain Res, 163: 299–318
Höglinger G U, Rizk P, Muriel M P, Duyckaerts C, OertelWH, Caille I, Hirsch E C (2004). Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci, 7(7): 726–735
Ito R, Robbins T W, Pennartz C M, Everitt B J (2008). Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci, 28(27): 6950–6959
Kwon O B, Paredes D, Gonzalez C M, Neddens J, Hernandez L, Vullhorst D, Buonanno A (2008). Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci USA, 105(40): 15587–15592
Levy G, Schupf N, Tang M X, Cote L J, Louis E D, Mejia H, Stern Y, Marder K (2002). Combined effect of age and severity on the risk of dementia in Parkinson’s disease. Ann Neurol, 51(6): 722–729
Loughlin S E, Foote S L, Bloom F E (1986). Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience, 18 (2): 291–306
Loy R, Koziell D A, Lindsey J D, Moore R Y (1980). Noradrenergic innervation of the adult rat hippocampal formation. J Comp Neurol, 189(4): 699–710
Meibach R C, Siegel A (1977). Efferent connections of the hippocampal formation in the rat. Brain Res, 124(2): 197–224
Pohle W, Ott T, Müller-Welde P (1984). Identification of neurons of origin providing the dopaminergic innervation of the hippocampus. J Hirnforsch, 25(1): 1–10
Regensburger M, Prots I, Winner B (2014). Adult hippocampal neurogenesis in Parkinson’s disease: impact on neuronal survival and plasticity. Neural Plast, 2014: 454696
Reymann K, Pohle W, Müller-Welde P, Ott T (1983). Dopaminergic innervation of the hippocampus: evidence for midbrain raphe neurons as the site of origin. Biomed Biochim Acta, 42(10): 1247–1255
Rosen Z B, Cheung S, Siegelbaum S A (2015). Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat Neurosci, 18(12): 1763–1771
Samuels E R, Szabadi E (2008). Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Curr Neuropharmacol, 6(3): 235–253
Sara S J (2009). The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci, 10(3): 211–223
Scatton B, Simon H, Le Moal M, Bischoff S (1980). Origin of dopaminergic innervation of the rat hippocampal formation. Neurosci Lett, 18(2): 125–131
Schwab M E, Javoy-Agid F, Agid Y (1978). Labeled wheat germ agglutinin (WGA) as a new, highly sensitive retrograde tracer in the rat brain hippocampal system. Brain Res, 152(1): 145–150
Schwarz L A, Miyamichi K, Gao X J, Beier K T, Weissbourd B, De Loach K E, Ren J, Ibanes S, Malenka R C, Kremer E J, Luo L (2015). Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature, 524(7563): 88–92
Seib D R, Corsini N S, Ellwanger K, Plaas C, Mateos A, Pitzer C, Niehrs C, Celikel T, Martin-Villalba A (2013). Loss of Dickkopf-1 restores neurogenesis in old age and counteracts cognitive decline. Cell Stem Cell, 12(2): 204–214
Simon H, Le Moal M, Calas A (1979). Efferents and afferents of the ventral tegmental-A10 region studied after local injection of [3H] leucine and horseradish peroxidase. Brain Res, 178(1): 17–40
Small S A, Schobel S A, Buxton R B, Witter M P, Barnes C A (2011). A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci, 12(10): 585–601
Smith C C, Greene R W (2012). CNS dopamine transmission mediated by noradrenergic innervation. J Neurosci, 32(18): 6072–6080
Spalding K L, Bergmann O, Alkass K, Bernard S, Salehpour M, Huttner H B, Boström E, Westerlund I, Vial C, Buchholz B A, Possnert G, Mash D C, Druid H, Frisén J (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell, 153(6): 1219–1227
Sui Y, Horne M K, Stanic D (2012). Reduced proliferation in the adult mouse subventricular zone increases survival of olfactory bulb interneurons. PLoS ONE, 7 (2): e31549
Suzuki K, Okada K, Wakuda T, Shinmura C, Kameno Y, Iwata K, Takahashi T, Suda S, Matsuzaki H, Iwata Y, Hashimoto K, Mori N (2010). Destruction of dopaminergic neurons in the midbrain by 6- hydroxydopamine decreases hippocampal cell proliferation in rats: reversal by fluoxetine. PLoS ONE, 5 (2): e9260
Swanson LW (1982). The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull, 9(1-6): 321–353
Szabadi E (2013). Functional neuroanatomy of the central noradrenergic system. J Psychopharmacol, 27(8): 659–693
Verney C, Baulac M, Berger B, Alvarez C, Vigny A, Helle K B (1985). Morphological evidence for a dopaminergic terminal field in the hippocampal formation of young and adult rat. Neuroscience, 14(4): 1039–1052
Wisman L A, Sahin G, Maingay M, Leanza G, Kirik D (2008). Functional convergence of dopaminergic and cholinergic input is critical for hippocampus-dependent working memory. J Neurosci, 28 (31): 7797–7807
Wyss J M, Swanson LW, Cowan W M (1979). A study of subcortical afferents to the hippocampal formation in the rat. Neuroscience, 4(4): 463–476
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Ermine, C.M., Wright, J.L., Parish, C.L. et al. Combined immunohistochemical and retrograde tracing reveals little evidence of innervation of the rat dentate gyrus by midbrain dopamine neurons. Front. Biol. 11, 246–255 (2016). https://doi.org/10.1007/s11515-016-1404-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11515-016-1404-4