Abstract
The B-type Raf kinase (BRAF) V600E mutation is a well-established biomarker for poor prognosis in metastatic colorectal cancer (mCRC) and is a highly attractive drug target. A barrier to the development of new therapies targeting BRAF V600E in mCRC is the low prevalence of mutations (approximately 10 %) and the current need for access to sequencing-based technologies which are not routinely available outside of large cancer centres. Availability of a standardised immunohistochemistry (IHC) test, more suited to routine pathology practice, would provide much broader access to patient identification. We sought to evaluate the accuracy and clinical utility of a recently developed BRAF V600E IHC method as a prognostic biomarker in a large cohort of community-based CRC patients. Archival tumour samples from 505 patients with stage I–IV CRC were immunohistochemically tested with two antibodies, pBR1 for total BRAF and VE1 for BRAF V600E. Cases were assessed by two blinded pathologists, and results were compared to BRAF V600E mutation status determined using DNA sequencing. Discordant cases were retested with a BRAF V600E SNaPshot assay. BRAF mutation status was correlated with overall survival (OS) in stage IV CRC. By DNA sequencing and IHC, 505 and 477 patients were respectively evaluable. Out of 477 patients, 56 (11. 7 %) had BRAF V600E mutations detected by sequencing and 63 (13.2 %) by IHC. Using DNA sequencing results as the reference, sensitivity and specificity for IHC were 98.2 % (55/56) and 98.1 % (413/421), respectively. IHC had a positive predictive value (PPV) of 87.3 % (55/63) and a negative predictive value (NPV) of 99.8 % (413/414). Compared to DNA sequencing plus retesting of available discordant cases by SNaPshot assay, IHC using the VE1 antibody had a 100 % sensitivity (59/59), specificity (416/416), NPV (416/416) and PPV (59/59). Stage IV CRC patients with BRAF V600E protein detected by IHC exhibited a significantly shorter overall survival (hazard ratio = 2.20, 95 % CI 1.26–3.83, p = 0.005), consistent with other published series. Immunohistochemistry using the BRAF V600E VE1 antibody is an accurate diagnostic assay in CRC. The test provides a simple, clinically applicable method of testing for the BRAF V600E mutation in routine practice.
Similar content being viewed by others
Introduction
A serine/threonine kinase of the MAPK-ERK signalling pathway, B-type Raf kinase (BRAF), and other RAF family members are usually activated by GTP-bound RAS signalling downstream of the epidermal growth factor receptor (EGFR) or in response to other mitogens [1]. Mutant BRAF, however, displays constitutive activation when affected by missense mutation [2], most commonly V600E. Approximately 10 % of colorectal cancers (CRCs) harbour BRAF V600E [2–4], and this subset is associated with a significantly poorer survival [5–7] in patients with metastatic disease. BRAF mutation may also predict lack of benefit from anti-EGFR therapy [8, 9] in metastatic CRC, although reports are conflicting [10]. The current clinical value of BRAF V600E detection is the delineation of hereditary non-polyposis colorectal cancer (HNPCC)-associated tumours (BRAF wild type) from sporadic CRCs (BRAF V600E mutant) in mismatch repair-deficient colorectal disease [11–13] and rational patient enrolment to clinical trials testing BRAF inhibitors [6].
Various methods of genotyping tumour samples for BRAF status are currently used in diagnostic and research laboratories, ranging from traditional Sanger sequencing [14] to quantitative pyrosequencing [15], mutation-specific real-time polymerase chain reaction (RT-PCR) assays [16] and mass spectrometry-based methods [17]. Common to all these methods, however, is the requirement for DNA extraction from tissue and the need for rigorous protocols to minimise the impact of contamination of non-tumour cells on the overall tumour to non-tumour cell ratio.
Importantly, at this point in time, the expertise and infrastructure required for DNA-based genotyping methods are frequently available only at academic centres and reference laboratories. Testing for BRAF V600E therefore requires multiple steps and coordination between the primary site and reference laboratory, resulting in sample transit costs and diagnostic delays. Until genomic-based testing becomes available in routine community-based pathology laboratories, the complexity involved in such testing will continue to serve as an impediment to providing a patient’s BRAF status to their treating clinician.
A monoclonal antibody specific to the BRAF V600E kinase, VE1, has recently been described [18] and offers the advantages of immunohistochemical determination of tumour BRAF mutation status, no requirement for DNA purification, low cost and the ability to perform testing on formalin-fixed paraffin-embedded (FFPE) tissue in routine histopathology laboratories. To date, immunohistochemistry with VE1 has been applied to the detection of BRAF V600E in brain metastases of varied primary sites [19], papillary thyroid carcinoma [20, 21], Langerhans cell histiocytosis [22, 23], ovarian carcinomas [24, 25], melanoma [26–28], lung adenocarcinoma [29] and hairy cell leukemia [30]. A recently published study by Sinicrope et al. explored the VE1 antibody in a carefully preselected group of 75 patients with stage III colorectal cancer, for whom BRAF mutation status had already been determined [31]. In another recent study examining the utility of BRAF immunohistochemistry (IHC) in microsatellite unstable CRC, Toon et al. [32] compared BRAF IHC with conventional PCR-based molecular methods for BRAF V600E detection in 216 patients with CRC. In a further cohort, they also performed IHC to mismatch repair (MMR) proteins and BRAF V600E in a larger cohort of 1,403 patients with CRC but failed to also validate this using conventional sequencing-based molecular techniques.
In this study, we aimed to determine the sensitivity, specificity and predictive values of VE1 immunohistochemistry for BRAF V600E in a large community-based and unselected cohort (n = 505) of patients with CRC, with the intent of determining how this could inform the use of this IHC-based antibody in routine practice. FFPE tumour samples were annotated for clinical outcomes and had been previously assessed for BRAF status by direct (Sanger) sequencing.
Methods
Colorectal tissue samples
Primary tumour and matched normal tissue samples were obtained from an unselected community-based cohort of 505 patients with CRC undergoing surgery at three hospitals in Melbourne, Australia: the Royal Melbourne, Melbourne Private and Western Hospitals. Resected tumours included those from the proximal colon, distal colon and rectum, and all disease stages (I–IV) were represented. This study was approved by the ethics committees of these hospitals and the University of Melbourne.
Tissue microarrays (TMAs) comprised of 1-mm-diameter tissue cores were constructed from the FFPE surgical specimens. Up to four tumour and two normal colon tissue cores were embedded per patient. Tumour cores were harvested from the areas of densest tumour cell percentage. Based on examination of hematoxylin and eosin (H&E)-stained TMA sections by two anatomical pathologists, cores were deemed to contain sufficient tumour sample for immunohistochemical analysis in 491 patients (97 %) (Fig. 1).
Sanger sequencing
All patients of the cohort were characterised for BRAF codon 600 mutation status based on Sanger sequencing at the Ludwig Institute Parkville, Melbourne, prior to immunohistochemical analysis of TMAs. Tumours were microdissected from the originating FFPE blocks, and the purified DNA was subjected to PCR and sequencing using primers and methods previously described [6]. Samples with indeterminate sequencing traces on first analysis were subjected to repeat PCR and sequencing.
Immunohistochemistry
Total BRAF and BRAF V600E-mutant proteins were detected by immunohistochemistry using rat anti-panBRAF monoclonal antibody (clone pBR1) and mouse anti-BRAF V600E monoclonal antibody (clone VE1), respectively, both kindly provided by Dr A. von Deimling from Ruprecht-Karls-University, Heidelberg [18]. All assays were performed on a BenchMark XT automated slide stainer at Ventana Medical Systems, Inc., Tucson, AZ.
Sections from TMAs were freshly cut to 4 μm and dried at 80 °C for 15 min. The presence of total BRAF protein was detected using ultraView Universal DAB Detection Kit (Ventana) where ultraView Universal HRP Multimer was substituted for an HRP-conjugated goat anti-rat secondary antibody. The staining procedure included deparaffinisation, pretreatment using standard Cell Conditioning 1, incubation with pan-BRAF antibody (diluted 1:8) at 37 °C for 16 min and treatment with ultrawash.
The BRAF V600E mutation-specific IHC assay was completed with OptiView DAB IHC Detection Kit (Ventana). Briefly, the tissue sections were deparaffinised, heat pretreated in Cell Conditioning 1 for 64 min and followed by inactivation of the endogenous peroxidases. Specimens were incubated with VE1 hybridoma supernatant (diluted 1:3) at 37 °C for 16 min.
Following the chromogenic detection, all slides were counterstained with Hematoxylin II and Bluing Reagent (Ventana) for 4 min each and coverslips were applied.
The immmunostained slides were evaluated independently by two pathologists (S.S. and D.W.) blinded to the BRAF V600E mutation status as determined by sequencing. First, pan-BRAF IHC was assessed for the presence of total BRAF cytoplasmic staining within invasive tumour cells. Cases were scored as unevaluable when total BRAF expression could not be detected, and they were excluded from further immunohistochemical analysis. Next, BRAF V600E (VE1) immunostained slides were evaluated for the presence or absence of BRAF V600E protein expression. Immunoreactivity was scored positive when there was unequivocal cytoplasmic staining above background in the majority of invasive viable tumour cells. Any nuclear staining, weak cytoplasmic staining of isolated tumour cells or focal confluent staining of tumour cells in a tumour that otherwise showed no staining was scored as immunonegative.
SNaPShot assays
DNA was extracted with QIAamp DNA FFPE Tissue Kit (Qiagen) from tumour cells microdissected from unstained FFPE tissue slides and then quantitated using a Qubit® fluorometer (Invitrogen, Grand Island, NY, USA). Purified tumour DNA was PCR-amplified for 30 cycles at 94 and 72 °C, for 45 s each, using BRAF exon 15 forward (TTCATAATGCTTGCTCTGATAGG) and reverse (AGTAACTCAGCAGCATCTCAGG) primers (GeneWorks, Thebarton, SA, Australia) and AmpliTaq Gold® DNA Polymerase (Applied Biosystems, Grand Island, NY, USA). The 246-bp products were treated with ExoSAP-IT (USB) at 37 °C for 30 min and 80 °C for 15 min to remove excess nucleotides and primers. HPLC-purified detection primer ((C)5TGATTTTGGTCTAGCTACAG) (GeneWorks) was added to the cleaned product together with PRISM SNaPshot Multiplex Ready Reaction Mix (Applied Biosystems) containing fluorescent ddNTPs. The detection primer was extended by thermocyling for 35 cycles at 96 °C for 10 s, 48 °C for 1 min and then 60 °C for 30 s. Excess nucleotides and primers were removed by shrimp alkaline phosphatase (Sigma, St. Louis, MO, USA) treatment at 37 °C for 1 h followed by 75 °C for 15 min, and the cleaned fragments were run in Hi-Di Formamide (Applied Biosystems) through an ABI 3130xl genetic analyser (Applied Biosystems) and analysed by GeneMapper® fragment analysis software. A mutation was called when the area under the fluorescent peak for the mutant allele was greater than five times the background relative fluorescent units. This method has been reported to detect mutations in tissue samples containing ≤5 % tumour cells [33–35].
Statistical analyses
Sensitivity, specificity and predictive values for IHC in comparison to direct DNA sequencing were determined as per convention [36, 37]. Overall survival (OS) in stage IV disease was defined as the time from diagnosis of metastatic CRC to death from any cause. Analyses included patients with de novo stage IV disease and those diagnosed with distance recurrence after prior treatment of earlier stage disease. Survival times were estimated using the Kaplan-Meier method, and results were compared using the log-rank test. Statistical significance was defined as p < 0.05.
Results
BRAF V600E status
Using Sanger sequencing, 11.7 % (59/505) of stage I–IV CRC patients were BRAF V600E mutation positive and the remainder were wild-type (WT). No tumour carried non-V600E mutations at codon 600. Of the 491 patients with adequate TMA tumour tissue for interpretation of total BRAF (pBR1 antibody) and BRAF V600E (VE1 antibody) immunohistochemistry, 14 patients (2.9 %) were negative for pBR1 staining and were accordingly deemed unevaluable for BRAF status by IHC (Fig. 1). Of the 477 ‘evaluable’ (pBR1 positive) patients, 63 (13.2 %) were positive for VE1 (‘BRAF V600E’) and 414 (86.8 %) were negative for VE1 staining (‘BRAF WT’) (Fig. 2). Of the 63 pBR1- and VE1-positive cases, the majority showed moderate to strong homogeneous cytoplasmic staining with the VE1 antibody. Occasional cases showed uniformly weak staining or focal patches of no staining in otherwise clearly positive tumours. Of the 414 pBR1-positive and VE1-negative cases, 8 (1.9 %) showed weak patchy positivity for VE1 (Fig. 3). All eight such heterogeneous cases were BRAF WT by sequencing. In normal colonic mucosa, nuclear VE1 staining was frequently observed in surface epithelial cells (Fig. 4).
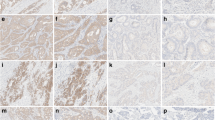
Representative images of pBR1-positive tumours either positive or negative for VE1. The upper panels represent a BRAF wild-type tumour, showing a hematoxylin and eosin (H&E), b total BRAF (pBR1) expression and c lack of BRAF V600E (VE1) protein. The lower panels demonstrate a confirmed BRAF V600E mutant tumour displaying d H&E and e total BRAF (pBR1) and f BRAF V600E (VE1) protein expression
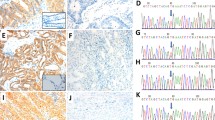
Representative images demonstrating heterogeneous staining of mutant BRAF V600E protein. a–d pan-BRAF IHC (pBR1) detects total BRAF within all cores of a single case. e–h Using the mutation-specific antibody VE1, mutant BRAF V600E is only present within cores f and h and absent within cores e and g of the same case
The clinicopathological features and IHC results for the evaluable patient population are shown in Table 1. A higher frequency of BRAF V600E positivity was seen in female patients and CRCs resected from the right colon, exhibiting poor differentiation or with microsatellite instability (MSI). These results are consistent with the findings of multiple prior cohort studies typing V600 mutations using DNA-based methods [38–40].
The sensitivity, specificity and predictive values for IHC were determined in the evaluable population as compared to the results from Sanger sequencing, which was considered to be the current gold standard for the determination of BRAF genotype. The sensitivity of VE1 staining for BRAF V600E was 98.2 % (55/56), specificity 98.1 % (413/421), negative predictive value (NPV) 99.8 % (413/414) and positive predictive value (PPV) 87.3 % (55/63) (Table 2).
Further investigation of discordant cases
Based on the Sanger sequencing results and IHC conducted on TMA cores, there were eight apparent IHC false-positive cases and one apparent IHC false-negative case (Table 2, Fig. 5). We sought to resolve these nine discordant cases by retesting and applying additional methods to detect or exclude BRAF V600E. To exclude the possibilities of sample mismatches or sampling error arising from the use of TMA cores for IHC, whole tissue sections were cut from the original FFPE tissue blocks and reprocessed for BRAF sequencing and IHC. In addition, to increase the sensitivity of DNA-based determination of BRAF mutation status, BRAF SNaPshot assays [34, 35] were performed on DNA extracted from tumour cells microdissected from sections adjacent to the original tissue sections utilised.
The original archival FFPE tissue blocks could be obtained for these further analyses from seven of the nine patients with discordant results, comprising six IHC false-positive and the single IHC-false negative case (Fig. 5). For three of the initial IHC false-positive cases, reanalyses confirmed the presence of the BRAF V600E mutation upon IHC and SNaPshot (allele frequencies of 33, 27 and 3 %), but Sanger sequencing again missed these mutant cases. In contrast, the other three initial IHC false-positive cases were found to be WT upon reanalysis with Sanger sequencing, SNaPshot and IHC on the whole mount slides (two with 0 % and one with 10 % of cells staining). Of the three cases erroneously VE1 positive on TMA IHC, review of the tissue core harvest sites on the VE1 whole sections showed sampling from heterogeneously stained areas in two of the three cases.
The single initial IHC false-negative case was confirmed to be BRAF V600E by resequencing and SNaPshot analysis (mutant allele frequency of 17 %) and showed corresponding VE1-positive staining in 47 % of tumour cells in the whole tissue section. Most discrepancies were, therefore, due to either low sensitivity of Sanger sequencing and/or heterogeneous VE1 antibody staining compounded by sampling error from the use of TMAs. When the two discordant cases with unavailable blocks for retesting were excluded, the VE1 antibody had a 100 % sensitivity, specificity, NPV and PPV in the population of reevaluable cases (n = 475) (Table 3).
Prognostic significance of VE1 positivity
We determined the prognostic significance of VE1 positivity in patients of the evaluable cohort with de novo (n = 108) or recurrent (n = 87) metastatic disease using final results after investigation of the discordant cases with whole mount IHC and SNaPShot assays. Survival data was available for 188 (96 %) patients of this subgroup. The median OS with metastatic disease for patients with BRAF V600E tumours on IHC was 275 days compared to 518 days for those with BRAF WT. The hazard ratio for OS with BRAF V600E was 2.20 (95 % CI 1.26–3.83, p = 0.005) (Fig. 6).
Discussion
Mutation of BRAF V600E is a well-validated poor prognostic marker in metastatic CRC [5–7], as well as a discriminator between HNPCC-related disease and sporadic MSI CRCs [11–13]. Perhaps the greatest promise of BRAF V600E detection, however, is in the application of targeted therapies in the emerging era of personalised medicine. While BRAF-mutant colorectal cancer has not demonstrated the same responsiveness to single-agent BRAF inhibitors seen in metastatic melanoma [41, 42], recent research findings show encouraging headway in the characterisation of CRC resistance mechanisms [42–44]. Clinical studies combining BRAF inhibitors with EGFR inhibitors, PI3K inhibitors or MEK inhibitors are now underway based on these recent data and will require accurate, efficient identification of patients with BRAF-mutant CRC.
We report a large series of community-based colorectal cancers screened for BRAF V600E mutation using the novel, mutation-specific monoclonal antibody VE1. In this study, blinded interpretation of VE1 IHC on CRC cores embedded in TMAs showed 98.2 % sensitivity, 98.1 % specificity, 87.3 % PPV and 99.8 % NPV against BRAF genotype determined by direct sequencing. Similar sensitivities and specificities for VE1 have been reported in papillary thyroid cancer, lung cancer and melanoma [20, 27–29]. Given that positive IHC results are likely to be verified by gene-based mutation detection methods and accurate identification of BRAF mutation is expected to facilitate access to novel targeted therapies, the NPV of VE1 IHC is of the highest importance. Only one sample in this study was falsely negative on VE1 TMA IHC and subsequently shown to be clearly VE1 positive on whole-section staining. The resolution of discordant findings for this case, and the further six available TMA IHC false-positive cases, by additional methods is reasonable in the context of this particular study. It is important to note that whole-section IHC would be conducted in routine clinical practice, rather than via the creation of TMAs. In addition, numerous genotyping methods have been recently demonstrated to be more sensitive than Sanger sequencing [15–17, 27]. The sensitivity, specificity, PPV and NPV were each recalculated as 100 % in the fully evaluable patient population. As expected, clinical correlations with BRAF V600E positivity confirmed the same patient and tumour associations in this patient cohort seen with BRAF genotype as determined by DNA-based mutation detection methods [38–40], further supporting the validity of this diagnostic approach in community-based patient cohorts. Although we restricted survival data to the subgroup of patients with metastatic disease (either de novo stage IV or recurrent stage IV disease, n = 188), given this is the group of patients for which the presence of a BRAF V600E mutation is a clear prognostic factor, our survival data was again consistent with that seen in other series.
In studies forerunning to those using anti-BRAF antibodies, the ability to apply immunohistochemistry for the detection of mutated gene products has been recognised in the management of gliomas. A monoclonal antibody specific for the mutant protein IDH1 R132H, important diagnostically and prognostically [45], has been developed and applied to clinical samples [46, 47]. Similarly, determination of BRAF mutation status by IHC is anticipated to confer significant benefits in the clinical setting. Unlike genotyping methods, IHC is routinely performed in all hospital histopathology departments. The cost of reagents, equipment and labour is projected to be lower than that of currently available sequencing methods. Additionally, the time to results may be shorter due to the on-site sample processing and absence of requirement for DNA extraction. Routine profiling of all newly diagnosed CRCs for BRAF V600E mutation may therefore now be feasible with an inexpensive and widely available methodology. This takes on added significance when considering BRAF V600E’s relatively low population prevalence. Detection of BRAF V600E mutation at the time of primary CRC resection and histopathological analysis has the advantages of excluding underlying HNPCC in MSI CRCs [11–13], with resultant screening implications [48], and prevention of lag time awaiting BRAF mutation results in patients later diagnosed with metastatic disease. Given that patients with metastatic BRAF-mutant CRC have a significantly poorer prognosis, early recognition of their BRAF status may alter clinical decisions regarding disease observation and, in the future, administration of molecularly directed therapies.
As per the original description of VE1 IHC on clinical samples [18], this study applied both VE1 and pBR1 primary antibodies to all tumours. While VE1 had been selected from 2,234 hybridoma clones as the only immunoglobulin specific for BRAF V600E on IHC, pBR1 had been chosen from pan-BRAF clones for its demonstrated ability to bind both BRAF wild type and BRAF V600E on IHC and Western blots [18]. The utility of positive pBR1 staining is in its confirmation of BRAF protein expression, reducing the likelihood of false-negative VE1 IHC findings, i.e. the presence of BRAF V600E genotype but negative VE1 staining due to the absence of BRAF protein expression in the tumour. In this cohort, only 14 of 491 primary CRCs (2.9 %) were negative on pBR1 IHC, suggesting that failure to express BRAF is rare in CRC and the routine use of pBR1 concurrently with VE1 may be unnecessary for this tumour type. More important than concurrent pBR1 staining may be preservation of tissue quality, as tissue areas subject to diathermy, showing necrosis, or frozen and then formalin-fixed have all been reported to show lower antigenicity for VE1 [18]. The use of freshly cut tissue sections is also recommended [19]. We noted strong staining of normal colonic surface epithelial cell nuclei for VE1, which could be used as a positive internal control for VE1 IHC protocols. The reason for nuclear staining with the VE1 antibody is unknown.
Immunohistochemistry may be an optimal method for determining the BRAF V600E mutation status of tissue samples with low cellularity due to its single-cell-level resolution. In contrast, for DNA-based detection methods, even microdissected tissue samples contain significant proportions of stromal cell-derived DNA, posing challenges in the detection of diluted tumour cell-derived mutant alleles. BRAF V600E-specific IHC has some limitations, however. Samples collected by fine-needle aspiration (FNA) may pose a challenge because of the fixatives used. Caution is urged until the validity of this approach in FNA samples has been adequately investigated. Due to the heterogeneity of VE1 staining seen in a proportion of CRCs, 1.7 % of the evaluable population in this study, small biopsy (e.g. core biopsies) and tissue specimens may be erroneously interpreted due to sampling error. This phenomenon was documented in our patient cohort with the initial use of TMAs for VE1 IHC; for one sample, IHC findings were changed from positive to negative, and for three samples, the reverse occurred when TMA IHC findings were compared to staining on whole mount sections. Although in our patient cohort <3 % of CRCs were negative on pan-BRAF IHC, other malignancies with low BRAF protein expression may not be amenable to IHC testing. VE1 is highly specific for BRAF V600E and does not detect the protein products of other BRAF aa.600 mutations [19, 27, 28]; hence for diseases with a higher burden of BRAF non-V600E mutations, DNA-based genotyping or alternative monoclonal antibodies may be indicated, particularly should these mutations demonstrate drug sensitivity. In CRC, <4 % of BRAF mutations are non-V600E [4, 9], and no other aa.600 mutations were detected by sequencing in the 505 patients of this cohort. However, we cannot exclude the possibility that a small number of patients may have carried other non-aa.600 BRAF mutations, as DNA sequencing was limited to the region of aa.600.
Two recently published studies exploring the VE1 antibody in colorectal cancer have also shown a high degree of specificity and sensitivity, but included either small series [32] and/or a highly preselected patient population [31]. However, the consistent findings in both of these studies add considerable weight to our findings in supporting the incorporation of the antibody as the initial method of detection of BRAF V600E status in patients with colorectal cancer. As opposed to the findings of strong concordance between the VE1 IHC antibody and sequencing-based techniques in these two studies and ours, another study examining VE1 IHC in colorectal cancer was also published recently and reports low sensitivity (71 %) and specificity (74 %) of VE1 tested on 52 FFPE CRC samples [49]. Methodological differences in the antigen retrieval, staining and signal amplification protocols resulting in poor signal intensity and background staining likely account for this discrepancy [49]. We found that the use of OptiView DAB IHC Detection Kit resulted in clear discrimination between background tissue and BRAF V600E-positive tumour cell cytoplasmic staining using the VE1 antibody. In our experience, patchy focal staining, rather than the level of staining intensity, can lead to difficulties with interpretation, particularly in tissue core samples. Patchy focal staining was occasionally seen (1.7 %) in otherwise clearly negative tumours and could lead to false-positive results (Fig. 3). Patchy focal non-staining was occasionally seen in otherwise clearly positive tumours and could lead to false-negative results. These staining anomalies were often present in the same areas in restained sections and did not correspond to any anatomical features such as tumour nodules. Interpretive difficulties were often resolved by examining additional tumour areas by staining whole mount sections. In such cases, confirmation of BRAF status should be verified by IHC on a different specimen from that resection or by a sensitive molecular method.
The observation that the vast majority of CRCs stained in this study showed either homogeneously negative or positive BRAF V600E expression argues against the clonal acquisition of BRAF activating mutations in primary CRCs. Interestingly, lack of clonality for BRAF V600E expression has also been noted in other malignancies including metastatic lesions [19–21, 28], despite suggestions of polyclonality for BRAF genotype in melanoma and thyroid cancer using alternative experimental methods [50–52]. The use of mutation-specific antibodies to address this controversial cancer biology question is but one of their potential research applications. Busam et al. [53] and Sahm et al. [23] utilised VE1 IHC in co-staining experiments to further characterise BRAF mutation-positive melanocytic lesions and Langerhans cell histiocytosis respectively. Exploration of staining intensity has also been suggested as a potentially fruitful translational research application [54].
In conclusion, the BRAF V600E VE1 antibody is an accurate immunohistochemical diagnostic assay in patients with CRC and should serve as a simple method for the detection of the BRAF V600E mutation in routine practice.
References
Marshall CJ (1996) Ras effectors. Curr Opin Cell Biol 8:197–204
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R et al (2002) Mutations of the BRAF gene in human cancer. Nature 417:949–954
Simi L, Pratesi N, Vignoli M, Sestini R, Cianchi F, Valanzano R, Nobili S, Mini E, Pazzagli M, Orlando C (2008) High-resolution melting analysis for rapid detection of KRAS, BRAF, and PIK3CA gene mutations in colorectal cancer. Am J Clin Pathol 130:247–253
Fumagalli D, Gavin PG, Taniyama Y, Kim SI, Choi HJ, Paik S, Pogue-Geile KL (2010) A rapid, sensitive, reproducible and cost-effective method for mutation profiling of colon cancer and metastatic lymph nodes. BMC Cancer 10:101
Ogino S, Nosho K, Kirkner GJ, Kawasaki T, Meyerhardt JA, Loda M, Giovannucci EL, Fuchs CS (2009) CpG island methylator phenotype, microsatellite instability, BRAF mutation and clinical outcome in colon cancer. Gut 58:90–96
Tie J, Gibbs P, Lipton L, Christie M, Jorissen RN, Burgess AW, Croxford M, Jones I, Langland R, Kosmider S, McKay D, Bollag G, Nolop N et al (2011) Optimizing targeted therapeutic development: analysis of a colorectal cancer patient population with the BRAFV600E mutation. Int J Cancer 128:2075–2084
Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O, Desai J (2011) Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 117:4623–4632
Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Leconte T, Rougier P, Lievre A, Landi B, Boige C, Ducreux M, Ychou M, Bibeau F et al (2009) Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol 27:5924–5930
De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras K, Kotoula V, Papamichael D, Laurent-Puig P, Penault-Llorca F, Rougier P, Vincenzi B et al (2010) Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 11:753–762
Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne CH (2012) Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 48:1466–1475
Deng G, Bell I, Crawley S, Gum J, Terdiman JP, Allen BA, Truta B, Sleisenger MH, Kim YS (2004) BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res 10:191–195
Domingo E, Laiho P, Ollikainen M, Pinto M, Wang L, French AJ, Westra J, Frebourg T, Espín E, Armengol M, Hamelin R, Yamamoto H, Hofstra RMW et al (2004) BRAF screening as a low-cost effective strategy for simplifying HNPCC genetic testing. J Med Genet 41:664–668
Robinson KL, Liu T, Vandrovcova J, Halvarsson B, Clendenning M, Frebourg T, Papadopoulos N, Kinzler KW, Vogelstein B, Peltomäki P, Kolodner RD, Nilbert M, Lindblom A (2007) Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics. J Natl Cancer Inst 99:291–299
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci 47:5463–5467
Spittle C, Ward MR, Nathanson KL, Gimotty PA, Rappaport E, Brose MS, Medina A, Letrero R, Herlyn M, Edwards RH (2007) Application of a BRAF pyrosequencing assay for mutation detection and copy number analysis in malignant melanoma. J Mol Diagn 9:464–471
Halait H, Demartin K, Shah S, Soviero S, Langland R, Cheng S, Hillman G, Wu L, Lawrence HJ (2012) Analytical performance of a real-time PCR-based assay for V600 mutation in the BRAF gene, used as the companion diagnostic test for the novel BRAF inhibitor vemurafenib in metastatic melanoma. Diagn Mol Pathol 21:1–8
Thomas RK, Baker AC, DeBiasi RM, Winckler W, LaFramboise T, Lin WM, Wang M, Feng W, Zander T, MacConnaill LE, Lee JC, Nicoletti R, Hatton C et al (2007) High throughput oncogene mutation profiling in human cancer. Nat Genet 39:347–351
Capper D, Preusser M, Habel A, Sahm F, Ackermann U, Schindler G, Pusch S, Mechtersheimer G, Zentgraf H, von Deimling A (2011) Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol 122:11–19
Capper D, Berghoff AS, Magerle M, Ilhan A, Wöhrer A, Hackl M, Pichler J, Pusch S, Meyer J, Habel A, Petzelbauer P, Birner P, von Deimling A, Preusser M (2012) Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol 123:223–233
Bullock M, O’Neill C, Chou A, Clarkson A, Dodds T, Toon C, Sywak M, Sidhu SB, Delbridge LW, Robinson BG, Learoyd DL, Capper D, von Deimling A et al (2012) Utilisation of a monoclonal antibody for BRAFV600E detection in papillary thyroid carcinoma. Endocr Relat Cancer 19:779–784
Kopereck O, Kornauth C, Capper D, Berghoff AS, Sari R, Niederle B, von Deimling A, Birner P, Preusser M (2012) Immunohistochemical detection of the BRAF V600E-mutated protein in papillary thyroid carcinoma. Am J Surg Pathol 36:844–850
Haroche J, Charlotte F, Arnaud L, von Deimling A, Hélias-Rodzewicz Z, Hervier B, Cohen-Aubart F, Launay D, Lesot A, Mokhtari K, Canioni D, Galmiche L, Rose C et al (2012) High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood 120:2700–2703
Sahm F, Capper D, Preusser M, Meyer J, Stenzinger A, Lasitschka F, Berghoff AS, Habel A, Schneider M, Kulozik A, Anagnostopoulos I, Müllauer L, Mechtersheimer G et al (2012) BRAFV600E mutant protein is expressed in cells of variable maturation in Langerhans cell histiocytosis. Blood 120:e28–e34
Bösmüller H, Fischer A, Pham DL, Fehm T, Capper D, von Deimling A, Bonzheim I, Staebler A, Fend F (2013) Detection of the BRAF V600E mutation in serous ovarian tumours: a comparative analysis of immunohistochemistry with a mutation-specific monoclonal antibody and allele-specific PCR. Hum Pathol 44:329–335
Preusser M, Capper D, Berghoff AS, Horvat R, Wrba F, Schindl M, Schoppmann SF, von Deimling A, Birner P (2013) Expression of BRAF V600E mutant protein in epithelial ovarian tumors. Appl Immunohistochem Mol Morphol 21:159–164
Skorokhod A, Capper D, von Deimling A, Enk A, Helmbold P (2012) Detection of BRAF V600E mutations in skin metastases of malignant melanoma by monoclonal antibody VE1. J Am Acad Dermatol 67:488–491
Colomba E, Hélias-Rodzewicz Z, von Deimling A, Marin C, Terrones N, Pechaud D, Surel S, Côté JF, Peschaud F, Capper D, Blons H, Zimmermann U, Clerici T et al (2013) Detection of BRAF p.V600E mutations in melanomas. Comparison of four methods argues for sequential use of immunohistochemistry and pyrosequencing. J Mol Diagn 15:94–100
Long GV, Wilmott JS, Capper D, Preusser M, Zhang YE, Thompson JF, Kefford RF, von Deimling A, Scolyer RA (2013) Immunohistochemistry is highly sensitive and specific for the detection of V600E BRAF mutation in melanoma. Am J Surg Pathol 2013:61–65
Ilie M, Long E, Hofman C, Dadone B, Marquette CH, Mouroux J, Vignaud JM, Begueret H, Merlio JP, Capper D, von Deimling A, Emile JF, Hofman P (2013) Diagnostic value of immunohistochemistry for the detection of BRAFV600E mutation in primary lung adenocarcinoma Caucasian patients. Ann Oncol 24:742–748
Andrulis M, Penzel R, Weichert W, von Deimling A, Capper D (2012) Application of a BRAF V600E mutation-specific antibody for the diagnosis of hairy cell leukemia. Am J Surg Pathol 36:1796–1800
Sinicrope FA, Smyrk TC, Tougeron D, Thibodeau SN, Singh S, Muranyi A, Shanmugam K, Grogan TM, Alberts SR, Shi Q (2013) Mutation-specific antibody detects mutation BRAFV600E protein expression in human colon carcinomas. Cancer 119:2765–2770
Toon CW, Walsh MD, Chou A, Capper D, Clarkson A, Sioson L, Clarke S, Mead S, Walters RJ, Clendenning M, Rosty C, Young JP, Win AK et al (2013) BRAFV600E immunohistochemistry facilitates universal screening of colorectal cancers for Lynch Syndrome. Am J Surg Pathol 37:1592–1602
van Oers JMM, Lurkin I, van Exsel AJA, Nijsen Y, van Rhijn BWG, van der Aa MNM, Zwarthoff EC (2005) A simple and fast method for the simultaneous detection of nine fibroblast growth factor receptor 3 mutations in bladder cancer and voided urine. Clin Cancer Res 11:7743–7748
Lurkin I, Stoehr R, Hurst CD, van Tilborg AAG, Knowles MA, Hartmann A, Zwarthoff EC (2010) Two multiplex assays that simultaneously identify 22 possible mutation sites in the KRAS, BRAF, NRAS and PIK3CA genes. PLoS ONE 5:e8802
Magnin S, Viel E, Baraquin A, Valmary-Degano S, Kantelip B, Pretet JL, Mougin C, Bigand M, Girardo B, Borg C, Ferrand C (2011) A multiplex SNaPshot assay as a rapid method for detecting KRAS and BRAF mutations in advanced colorectal cancers. J Mol Diagn 13:485–492
Altman DG, Bland JM (1994) Diagnostic tests 1: sensitivity and specificity. BMJ 308:1552
Altman DG, Bland JM (1994) Diagnostic tests 2: predictive values. BMJ 309:102
Samowitz WS, Sweeney C, Herrick J, Albertsen H, Levin TR, Murtaugh MA, Wolff RK, Slatter ML (2005) Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res 65:6063–6070
Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B (2006) BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer 5:2
Kalady MF, Dejulius KL, Sanchez JA, Jarrar A, Liu X, Manilich E, Skacel M, Church JM (2012) BRAF mutations in colorectal cancer are associated with distinct clinical characteristics and worse prognosis. Dis Colon Rectum 55:128–133
Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, Chapman PB (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809–819
Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R (2012) Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483:100–104
Corcoran RB, Hiromichi E, Turke AB, Coffee EM, Nishino M, Cogdill AP, Brown RD, Della Pelle P, Dias-Santagata D, Hung KE, Flaherty KT, Piris A, Wargo JA et al (2012) EGFR-mediated reactivation of MAPK signalling contributes to insensitivity of BRAF-mutant colorectal cancers to RAF inhibitions with vemurafenib. Cancer Discov 2:227–235
Mao M, Tian F, Mariadason JM, Tsao CC, Lemos R, Dayyani F, Vashisht Gopal YN, Jiang ZQ, Wistuba II, Tang XM, Bornman WG, Bollag G, Mills GB et al (2013) Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res 19:657–667
Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W, Weller M, Herold-Mende C, Unterberg A et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendrial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118:469–474
Capper D, Weißert S, Balss J, Habel A, Meyer J, Jäger D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H, Hartmann C, von Deimling A (2010) Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol 20:245–254
Preusser M, Wöhrer A, Stary S, Höftberger R, Streubel B, Hainfellner JA (2011) Value and limitations of immunohistochemistry and gene sequencing for detection of the IDH1-R132H mutation in diffuse glioma biopsy specimens. J Neuropathol Exp Neurol 70:715–723
Lindor NM, Petersen GM, Hadley DW, Kinney AY, Miesfeldt S, Lu KH, Lynch P, Burke W, Press N (2006) Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome. A systematic review. JAMA 296:1507–1517
Adackapara CA, Sholl LM, Barletta JA, Hornick JL (2013) Immunohistochemistry using the BRAF V600E mutation-specific monoclonal antibody VE1 is not a useful surrogate for genotyping in colorectal adenocarcinoma. Histopathology 63:187–193
Lin J, Takata M, Goto Y, Kido K, Ferrone S, Saida T (2009) Polyclonality of BRAF mutations in acquired melanocytic nevi. J Natl Cancer Inst 101:1423–1427
Lin J, Goto Y, Murata H, Sakaizawa K, Uchiyama A, Saida T, Takata M (2011) Polyclonality of BRAF mutations in primary melanoma and the selection of mutant alleles during progression. Br J Cancer 104:464–468
Guerra A, Sapio MR, Marotta V, Campanile E, Rossi S, Forno I, Fugazzola L, Budillon A, Moccia T, Fenzi G, Vitale M (2012) The primary occurrence of BRAFV600E is a rare clonal event in papillary thyroid carcinoma. JCEM 97:517–524
Busam KJ, Sung J, Wiesner T, von Deimling A, Jungbluth A (2012) Combined BRAFV600E-positive melanocytic lesions with large epithelioid cells lacking BAP1 expression and conventional nevomelanocytes. Am J Surg Pathol 37:193–199
von Deimling A, Sahm F, Capper D (2012) Mutation specific antibodies: tool or dinosaur? Oncotarget 3:907–908
Acknowledgments
The authors thank the patients for participating in this study and the Victorian Cancer BioBank and Biogrid Australia for the provision of specimens and access to the clinical data, respectively.
Support
This study was supported by the Victorian Government through the Operational Infrastructure Support Program, the Victorian Cancer Agency and the Australian Government through the National Health Medical Research Council and Ventana Medical Systems, Inc. J.D. is supported by the Victorian Government through a Victorian Cancer Agency Clinical Researcher Fellowship. F.D. is supported by the Cancer Council Victoria through a Postgraduate Cancer Research Scholarship.
Conflict of interest
JD and PW received funding from Ventana Medical Systems, Inc., for this study to analyse clinical follow-up data and to perform the SNaPShot assays.
Author information
Authors and Affiliations
Corresponding author
Additional information
Paul Waring and Jayesh Desai contributed equally to this study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Day, F., Muranyi, A., Singh, S. et al. A mutant BRAF V600E-specific immunohistochemical assay: correlation with molecular mutation status and clinical outcome in colorectal cancer. Targ Oncol 10, 99–109 (2015). https://doi.org/10.1007/s11523-014-0319-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-014-0319-8