Summary
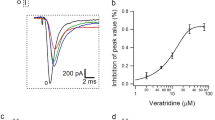
Neferine, a bisbenzylisoquinoline alkaloid in Lotus Plumule, was proved to have a wide range of biological activities. In the present study, using whole-cell patch-clamp technique, we investigated the effects of neferine on Nav1.5 channels that are stably expressed in HEK 293 cells. We found that neferine potently and reversibly inhibited Nav1.5 currents in a concentration dependent manner with a half-maximal inhibition (IC50) being 26.15 μmol/L. The inhibitory effects of neferine on Nav1.5 currents were weaker than those of quinidine at the same concentration. The steady-state inactivation curve was significantly shifted towards hyperpolarizing direction in the presence of 30 μmol/L neferine, while the voltage-dependent activation was unaltered. Neferine prolonged the time to peak of activation, increased the inactivation time constants of Nav1.5 currents and markedly slowed the recovery from inactivation. The inhibitory effect of neferine could be potentiated in a frequency-dependent manner. These results suggested that neferine can block Nav1.5 channels under the open state and inactivating state and it is an open channel blocker of Nav1.5 channels.
Similar content being viewed by others
References
Park DS, Fishman GI. Navigating through a complex landscape: Scn10a and cardiac conduction. J Clin Invest, 2014,124(4):1460–1462
Lei M, Zhang H, Grace AA, et al. Scn5a and sinoatrial node pacemaker function. Cardiovasc Res, 2007,74(3): 356–365
Goldin AL. Mechanisms of sodium channel inactivation. Curr Opin Neurobiol, 2003,13(3):284–290
Wang Y, Mi J, Lu K, et al. Comparison of gating properties and use-dependent block of nav1.5 and nav1.7 channels by anti-arrhythmics mexiletine and lidocaine. PLoS One, 2015,10(6):e0128653
Rogart RB, Cribbs LL, Muglia LK, et al. Molecular cloning of a putative tetrodotoxin-resistant rat heart na+ channel isoform. Proc Natl Acad Sci USA, 1989,86(20): 8170–8174
Schwartz PJ, Priori SG, Locati EH, et al. Long QT syndrome patients with mutations of the scn5a and herg genes have differential responses to na+ channel blockade and to increases in heart rate. Implications for gene-specific therapy. Circulation, 1995,92(12):3381–3386
Clancy CE, Rudy Y. Na(+) channel mutation that causes both brugada and long-qt syndrome phenotypes: A simulation study of mechanism. Circulation, 2002,105(10): 1208–1213
Benito B, Brugada R, Perich RM, et al. A mutation in the sodium channel is responsible for the association of long qt syndrome and familial atrial fibrillation. Heart Rhythm, 2008,5(10):1434–1440
Remme CA. Cardiac sodium channelopathy associated with scn5a mutations: Electrophysiological, molecular and genetic aspects. J Physiol, 2013,591(Pt17):4099–4116
Onkal R, Djamgoz MB. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: Clinical potential of neonatal nav1.5 in breast cancer. Eur J Pharmacol, 2009,625(1-3):206–219
Itoh A, Saitoh T, Tani K, et al. Bisbenzylisoquinoline alkaloids from nelumbo nucifera. Chem Pharm Bull, 2011,59(8):947–951
Yoon JS, Kim HM, Yadunandam AK, et al. Neferine isolated from nelumbo nucifera enhances anti-cancer activities in hep3b cells: Molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti-angiogenic response. Phytomedicine, 2013,20(11): 1013–1022
Gu DF, Li XL, Qi ZP, et al. Blockade of herg k+ channel by isoquinoline alkaloid neferine in the stable transfected hek293 cells. Naunyn Schmiedebergs Arch Pharmacol, 2009,380(2):143–151
Guo Z. Electrophysiological effects of neferine against ischemic ventricular tachyarrhythmias. Zhonghua Xin Xue Guan Bing Za Zhi (Chinese), 1992,20(2):119–122, 134
Li GR, Li XG, Lu FH. Effects of neferine on transmembrane potentials of guinea pig myocardium. Zhongguo Yao Li Xue Bao (Chinese), 1989,10(5):406–410
Wang C, Chen YF, Quan XQ, et al. Effects of neferine on kv4.3 channels expressed in hek293 cells and ex vivo electrophysiology of rabbit hearts. Acta Pharmacol Sin, 2015,36(12):1451–1461
Huang Y, Zhao L, Bai Y, et al. Simultaneous determination of liensinine, isoliensinine and neferine from seed embryo of nelumbo nucifera gaertn. In rat plasma by a rapid hplc method and its application to a pharmacokinetic study. Arzneimittelforschung, 2011,61(6):347–352
Poulin H, Bruhova I, Timour Q, et al. Fluoxetine blocks nav1.5 channels via a mechanism similar to that of class 1 antiarrhythmics. Mol Pharmacol, 2014,86(4):378–389
Ziyadeh-Isleem A, Clatot J, Duchatelet S, et al. A truncating scn5a mutation combined with genetic variability causes sick sinus syndrome and early atrial fibrillation. Heart Rhythm, 2014,11(6):1015–1023
Beyder A, Strege PR, Reyes S, et al. Ranolazine decreases mechanosensitivity of the voltage-gated sodium ion channel na(v)1.5: A novel mechanism of drug action. Circulation, 2012,125(22):2698–2706
Ju YK, Saint DA, Gage PW. Effects of lignocaine and quinidine on the persistent sodium current in rat ventricular myocytes. Br J Pharmacol, 1992,107(2):311–316
Kaufman ES. Quinidine in short qt syndrome: An old drug for a new disease. J Cardiovasc Electrophysiol, 2007,18(6):665–666
Snyders DJ, Hondeghem LM. Effects of quinidine on the sodium current of guinea pig ventricular myocytes. Evidence for a drug-associated rested state with altered kinetics. Circ Res, 1990,66(2):565–579
Bahring R, Covarrubias M. Mechanisms of closed-state inactivation in voltage-gated ion channels. J Physiol, 2011,589(Pt3):461–479
Wang H, Shi H, Zhang L, et al. Nicotine is a potent blocker of the cardiac a-type k(+) channels. Effects on cloned kv4.3 channels and native transient outward current. Circulation, 2000,102(10):1165–1171
Nishii N, Ogawa M, Morita H, et al. Scn5a mutation is associated with early and frequent recurrence of ventricular fibrillation in patients with brugada syndrome. Circ J, 2010,74(12):2572–2578
Giudicessi JR, Ye D, Tester DJ, et al. Transient outward current (i(to)) gain-of-function mutations in the kcnd3-encoded kv4.3 potassium channel and brugada syndrome. Heart Rhythm, 2011,8(7):1024–1032
Szel T, Antzelevitch C. Abnormal repolarization as the basis for late potentials and fractionated electrograms recorded from epicardium in experimental models of brugada syndrome. J Am Coll Cardiol, 2014,63(19):2037–2045
Mizusawa Y, Sakurada H, Nishizaki M, et al. Effects of low-dose quinidine on ventricular tachyarrhythmias in patients with brugada syndrome: Low-dose quinidine therapy as an adjunctive treatment. J Cardiovasc Pharmacol, 2006,47(3):359–364
He B, Soderlund DM. Human embryonic kidney (HEK293) cells express endogenous voltage-gated sodium currents and Nav 1.7 sodium channels. Neurosci Lett, 2010,469(2): 268–272
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Wang, H., Xiao, Jh. et al. Inhibitory effects of neferine on Nav1.5 channels expressed in HEK293 cells. J. Huazhong Univ. Sci. Technol. [Med. Sci.] 36, 487–493 (2016). https://doi.org/10.1007/s11596-016-1613-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-016-1613-8