Summary
Objective
The influential factors of chemotherapy-induced myelosuppression in esophageal cancer in central China are unclear. This study aimed to investigate the effect of commonly used chemotherapy regimens on the incidence of myelosuppression in clinical treatment of esophageal cancer.
Methods
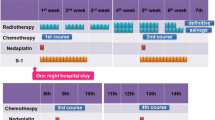
In this retrospective study, 624 patients with esophageal cancer who received six different chemotherapy regimens between 2013 and 2020 at our institute were included. Chemotherapy consisted of lobaplatin, 5-fluorouracil (5-F), lobaplatin and 5-F, nedaplatin, nedaplatin and paclitaxel (PTX), cisplatin and PTX. Multivariable logistic regression analysis was used to explore the risk of myelosuppression among the six different chemotherapy regimens.
Results
Compared with lobaplatin group, the incidence of myelosuppression in patients treated with chemotherapy regimens of lobaplatin and 5-F, nedaplatin, nedaplatin and PTX and cisplatin and PTX were significantly ameliorated. The dose of lobaplatin was significantly reduced (P=0.007) when lobaplatin was combined with 5-F, and the combination could significantly reduce the risk of myelosuppression (P=0.022). Furthermore, chemotherapeutic regimens, the dose of platinum, hemoglobin and uric acid levels, age, sex, total bilirubin and immune-enhancing drugs were found to be strong predictors of developing myelosuppression.
Conclusion
Targeted preventive interventions that enhance immune function, reduce uric acid levels and choose combined medication during chemotherapy should be implemented for high-risk patients to reduce the occurrence of myelosuppression. In addition, the dose of lobaplatin should be adjusted when combined with other chemotherapy drugs to reduce the incidence of myelosuppression.
Similar content being viewed by others
References
Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37513025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet (London, England), 2018,391(10125):1023–1075
Bollschweiler E, Plum P, Monig SP, et al. Current and future treatment options for esophageal cancer in the elderly. Expert Opin Pharmacother, 2017,18(10):1001–1010
Pickens A, Orringer MB. Geographical distribution and racial disparity in esophageal cancer. Ann Thorac Surg, 2003,76(4):S1367–1369
Bosetti C, Levi F, Ferlay J, et al. Trends in oesophageal cancer incidence and mortality in Europe. Int J Cancer, 2008,122(5):1118–1129
Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann oncol, 2016,27 (suppl 5):v50–v57
Ajani JA, J.S. Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw, 2011,9(8):830–887
Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today, 2020,50(1):12–20
Fatehi Hassanabad A, Chehade R, Breadner D, et al. Esophageal carcinoma: Towards targeted therapies. Cell Oncol (Dordr), 2020,43(2):195–209
Carr C, Ng J, Wigmore T. The side effects of chemotherapeutic agents. Curr Anaesth Crit Care, 2008,19(2):70–79
Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer, 2004,100(2):228–237
Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. J Natl Cancer Inst, 1999,91(19):1616–1634
Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology, 2015,29(4):282–294
Barreto JN, McCullough KB, Ice LL, et al. Antineoplastic agents and the associated myelosuppressive effects: a review. J Pharm Pract, 2014,27(5):440–446
Taylor SJ, Duyvestyn JM, Dagger SA, et al. Preventing chemotherapy-induced myelosuppression by repurposing the FLT3 inhibitor quizartinib. Sci Transl Med, 2017,9(402):eaam8060
Yin WJ, Yi YH, Guan XF, et al. Preprocedural Prediction Model for Contrast-Induced Nephropathy Patients. J Am Heart Assoc, 2017,6(2):e004498
Epstein RS, Aapro MS, Basu Roy UK, et al. Patient Burden and Real-World Management of Chemotherapy-Induced Myelosuppression: Results from an Online Survey of Patients with Solid Tumors. Adv Ther, 2020,37(8):3606–3618
Grading standard for acute and subacute toxicity of anticancer drugs (WHO standard), Cancer, 1992,(3):254
Saggam A, Kale P, Shengule S, et al. Ayurveda-based Botanicals as Therapeutic Adjuvants in Paclitaxel-induced Myelosuppression. Front Pharmacol, 2022,13: 835616
Zheng Y, Li Y, Liu X, et al. Multicentre Comparison of the Toxicity and Effectiveness of Lobaplatin-Based Versus Cisplatin-Based Adjuvant Chemotherapy in Oesophageal Carcinoma. Front Oncol, 2021,11:668140
Park MH, Jung IK, Min WK, et al. Neuropeptide Y improves cisplatin-induced bone marrow dysfunction without blocking chemotherapeutic efficacy in a cancer mouse model. BMB reports, 2017,50(8):417–422
Javarappa KK, D. Tsallos D, Heckman CA. A Multiplexed Screening Assay to Evaluate Chemotherapy-Induced Myelosuppression Using Healthy Peripheral Blood and Bone Marrow. SLAS Discov, 2018,23(7):687–696
Reddel CJ, Tan CW, Chen VM. Thrombin Generation and Cancer: Contributors and Consequences. Cancers, 2019,11(1):100
Sun W, Ren H, Gao CT, et al. Clinical and Prognostic Significance of Coagulation Assays in Pancreatic Cancer Patients With Absence of Venous Thromboembolism. American J Clin Oncol, 2015,38(6):550–556
Bestari MB, Agustanti N. Obstructive jaundice due to pancreatic metastasis from non-small cell lung cancer. Acta medica Indonesiana, 2013,45(3):216–219
Wang XB, Wu DJ, Chen WP, et al. Impact of radiotherapy on immunological parameters, levels of inflammatory factors, and clinical prognosis in patients with esophageal cancer. J Radiat Res, 2019,60(3):353–363
Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst, 2011,103(13):1049–1057
Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg, 2018, 41(3):210–215
Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977–2005. Brit J Cancer, 2009,101(5):855–859
Ohira M, Kubo N, Yamashita Y, et al. Impact of Chemoradiation-induced Myelosuppression on Prognosis of Patients with Locally Advanced Esophageal Cancer After Chemoradiotherapy Followed by Esophagectomy. Anticancer Res, 2015,35(9):4889–4895
Ojima T, Nakamori M, Nakamura M, et al. Phase I/II study of divided-dose docetaxel, cisplatin and fluorouracil for patients with recurrent or metastatic squamous cell carcinoma of the esophagus. Dis Esophagus, 2017,30(2):1–7
Lan YQ, Wu RP, Huang XB, et al. Paclitaxel, oxaliplatin, 5-fluorouracil and leucovorin combination chemotherapy in patients with recurrent or metastatic gastric cancer. Tumori, 2018,104(1):22–29
J. Liu, Z. Wang, Wu K, et al. Paclitaxel or 5-fluorouracil/esophageal stent combinations as a novel approach for the treatment of esophageal cancer. Biomaterials, 2015,53:592–599
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest Statement
The authors declare no conflicts of interest.
The present research was supported by a grant from the National Key R&D Programmes (NKPs) of China (No. 2017YFC0909900).
Rights and permissions
About this article
Cite this article
Zhang, Ql., Wu, Tt., Han, Y. et al. Chemotherapy-Induced Myelosuppression in Esophageal Cancer Patients: Risks and Suggestions for Its Management. CURR MED SCI 42, 530–537 (2022). https://doi.org/10.1007/s11596-022-2587-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2587-3