Abstract
Purpose
The performance of computer-aided detection (CAD) software depends on the quality and quantity of the dataset used for machine learning. If the data characteristics in development and practical use are different, the performance of CAD software degrades. In this study, we investigated changes in detection performance due to differences in training data for cerebral aneurysm detection software in head magnetic resonance angiography images.
Materials and methods
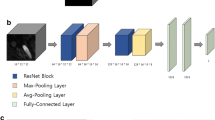
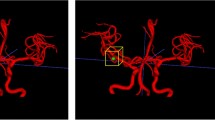
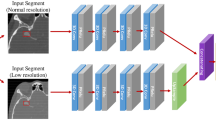
We utilized three types of CAD software for cerebral aneurysm detection in MRA images, which were based on 3D local intensity structure analysis, graph-based features, and convolutional neural network. For each type of CAD software, we compared three types of training pattern, which were two types of training using single-site data and one type of training using multisite data. We also carried out internal and external evaluations.
Results
In training using single-site data, the performance of CAD software largely and unpredictably fluctuated when the training dataset was changed. Training using multisite data did not show the lowest performance among the three training patterns for any CAD software and dataset.
Conclusion
The training of cerebral aneurysm detection software using data collected from multiple sites is desirable to ensure the stable performance of the software.





Similar content being viewed by others
References
Giger ML, Chan HP, Boone J. Anniversary paper: history and status of CAD and quantitative image analysis: the role of medical physics and AAPM. Med Phys. 2008;35(12):5799–820.
van Ginneken B, Schaefer-Prokop CM, Prokop M. Computer-aided diagnosis: how to move from the laboratory to the clinic. Radiology. 2011;261(3):719–32.
Litjens G, Kooi T, Bejnordi BE, et al. A survey on deep learning in medical image analysis. Med Image Anal. 2017;42:60–88.
Yasaka K, Akai H, Kunimatsu A, Kiryu S, Abe O. Deep learning with convolutional neural network in radiology. Jpn J Radiol. 2018;36(4):257–72.
Hu Z, Tang J, Wang Z, Zhang K, Zhang L, Sun Q. Deep learning for image-based cancer detection and diagnosis—a survey. Pattern Recogn. 2018;83:134–49.
Weikert T, Cyriac J, Yang S, Nesic I, Parmar V, Stieltjes B. A practical guide to artificial intelligence-based image analysis in radiology. Invest Radiol. 2020;55(1):1–7.
Bluemke DA, Moy L, Bredella MA, et al. Assessing radiology research on artificial intelligence: a brief guide for authors, reviewers, and readers–from the Radiology editorial board. Radiology. 2020;294(3):487–9.
Park SH, Han K. Methodologic guide for evaluating clinical performance and effect of artificial intelligence technology for medical diagnosis and prediction. Radiology. 2018;286(3):800–9.
Gibson E, Hu Y, Ghavami N, et al. Inter-site variability in prostate segmentation accuracy using deep learning. MICCAI 2018, LNCS. 2018;11073:506–14.
Onofrey JA, Casetti-Dinescu DI, Lauritzen AD, et al. Generalizable multi-site training and testing of deep neural networks using image normalization. In: 2019 IEEE 16th International Symposium on Biomedical Imaging (ISBI 2019), 2019, pp. 348–351.
Remedios SW, Roy S, Bermudez C, et al. Distributed deep learning across multisite datasets for generalized CT hemorrhage segmentation. Med Phys. 2020;47(1):89–98.
Therrien R, Doyle S. Role of training data variability on classifier performance and generalizability. Proc SPIE. 2018;10581:1058109.
Zech JR, Badgeley MA, Liu M, Costa AB, Titano JJ, Oermann EK. Variable generalization performance of a deep learning model to detect pneumonia in chest radiographs: a cross-sectional study. PLoS Med. 2018;15(11):e1002683.
Arimura H, Li Q, Korogi Y, et al. Automated computerized scheme for detection of unruptured intracranial aneurysms in three-dimensional magnetic resonance angiography. Acad Radiol. 2004;11(10):1093–104.
Kobashi SK, Konda K, Hata Y. Computer-aided diagnosis of intracranial aneurysms in MRA images with case-based reasoning. IEICE Trans Inf Syst. 2006;E89-D(1):340–50.
Yang X, Blezek DJ, Cheng LT, Ryan WJ, Kallmes DF, Erickson BJ. Computer-aided detection of intracranial aneurysms in MR angiography. J Digit Imaging. 2011;24(1):86–95.
Nomura Y, Masutani Y, Miki S, et al. Performance improvement in computerized detection of cerebral aneurysms by retraining classifier using feedback data collected in routine reading environment. J Biomed Graph Comput. 2014;4(4):12–21.
Hanaoka S, Nomura Y, Nemoto M, et al. HoTPiG: a novel geometrical feature for vessel morphometry and its application to cerebral aneurysm detection. MICCAI 2015, LNCS. 2015;9350:103–10.
Jin Z, Arimura H, Kakeda S, Yamashita F, Sasaki M, Korogi Y. An ellipsoid convex enhancement filter for detection of asymptomatic intracranial aneurysm candidates in CAD frameworks. Med Phys. 2016;43(2):951–60.
Nakao T, Hanaoka S, Nomura Y, et al. Deep neural network-based computer-assisted detection of cerebral aneurysms in MR angiography. J Magn Reson Imaging. 2018;47(4):948–53.
Sichtermann T, Faron A, Sijben R, Teichert N, Freiherr J, Wiesmann M. Deep learning-based detection of intracranial aneurysms in 3D TOF-MRA. Am J Neuroradiol. 2019;40(1):25–32.
Ueda D, Yamamoto A, Nishimori M, et al. Deep learning for MR angiography: automated detection of cerebral aneurysms. Radiology. 2019;290(1):187–94.
Hanaoka S, Nomura Y, Takenaga T, et al. HoTPiG: a novel graph-based 3-D image feature set and its applications to computer-assisted detection of cerebral aneurysms and lung nodules. Int J Comput Assist Radiol Surg. 2019;14(12):2095–107.
Joo B, Ahn SS, Yoon PH, et al. A deep learning algorithm may automate intracranial aneurysm detection on MR angiography with high diagnostic performance. Eur Radiol. 2020;30(11):5785–93.
Chen G, Wei X, Lei H, et al. Automated computer-assisted detection system for cerebral aneurysms in time-of-flight magnetic resonance angiography using fully convolutional network. Biomed Eng Online. 2020;19(1):38.
Hou W, Mei S, Gui Q, et al. 1D CNN-based intracranial aneurysms detection in 3D TOF-MRA. Complexity. 2020;2020:7023754.
Nyul LG, Udupa JK. On standardizing the MR image intensity scale. Magn Reson Med. 1999;42(6):1072–81.
Jiang X, Zhou Z, Ding X, Deng X, Zou L, Li B. Level set based hippocampus segmentation in MR images with improved initialization using region growing. Comput Math Methods Med. 2017;2017:5256346.
Koenderink JJ. Solid shape. Cambridge, MA: MIT Press; 1990.
Dorai C, Jain AK. COSMOS-a representation scheme for 3D free-form objects. IEEE Trans Pattern Anal Mach Intell. 1997;19(10):1115–30.
Nemoto M, Shimizu A, Hagihara Y, Kobatake H, Nawano S. Improvement of tumor detection performance in mammograms by feature selection from a large number of features and proposal of fast feature selection method. Syst Comput Jpn. 2006;37(12):56–68.
Li Q, Sone S, Doi K. Selective enhancement filters for nodules, vessels, and airway walls in two- and three-dimensional CT scans. Med Phys. 2003;30(8):2040–51.
Schapire RE, Freund Y, Bartlett P, Lee WS. Boosting the margin: a new explanation for the effectiveness of voting methods. Ann Statist. 1998;26(5):1651–86.
Kim K, Choi HI, Oh K. Object detection using ensemble of linear classifiers with fuzzy adaptive boosting. J Image Video Proc. 2017. https://doi.org/10.1186/s13640-017-0189-y.
Chakraborty DP, Berbaum KS. Observer studies involving detection and localization: modeling, analysis, and validation. Med Phys. 2004;31(8):2313–30.
Metz CE. Receiver operating characteristic analysis: a tool for the quantitative evaluation of observer performance and imaging systems. J Am Coll Radiol. 2006;3(6):413–22.
Setio AAA, Traverso A, de Bel T, et al. Validation, comparison, and combination of algorithms for automatic detection of pulmonary nodules in computed tomography images: the LUNA16 challenge. Med Image Anal. 2017;42:1–13.
Chang K, Balachandar N, Lam C, et al. Distributed deep learning networks among institutions for medical imaging. J Am Med Inform Assoc. 2018;25(8):945–54.
Nomura Y, Miki S, Hayashi N, et al. Novel platform for development, training, and validation of computer-assisted detection/diagnosis software. Int J Comput Assist Radiol Surg. 2020;15(4):661–72.
Acknowledgements
The authors would like to thank Dr. Shogo Nishiyama (Fuchinobe General Hospital, Kanagawa, Japan) for providing Dataset C. The Department of Computational Radiology and Preventive Medicine, The University of Tokyo Hospital, is sponsored by HIMEDIC Inc. and Siemens Healthcare K.K. This study was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number 19lk1010038h0001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest with regard to the present study.
Ethical approval
This study was approved by the ethical review boards of our institutions.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix: Methods used in CAD software
Shape index
The shape index [29, 30] represents a local feature of the surface. The value ranges from 0 to 1. The values of the voxels of a typical cerebral aneurysm range from 0.8 to 1.0, showing a peak or dome.
Selective enhancement filters derived from Hessian matrix
The Hessian matrix is a 3 × 3 square matrix composed of local second-order derivatives of the image. The selective enhancement filters for dot, line, and plane structures were derived from the eigenvalues of the Hessian matrix [32].
HoTPiG feature set
HoTPiG [18, 23] is a voxel-based feature set derived from a graph structure extracted from a binary image of the target structure (e.g., vessel system). The HoTPiG feature set is defined at each node (i.e., each voxel) in a given graph based on a 3D histogram of shortest path distances between the node of interest and each of its neighboring node pairs.
Mahalanobis distance ratio
The Mahalanobis distance is the distance between a point and a distribution. In the classification, first, the Mahalanobis distance dj (j = aneurysm, normal) between a feature vector at the target voxel and the multivariate distributions of two classes, which are estimated during training, are calculated. After that, the Mahalanobis distance ratio γ is calculated as follows:
If γ is above a certain threshold, the target voxel is classified as an aneurysm.
AdaBoost algorithm
AdaBoost is an adaptive algorithm to boost a sequence of classifiers [33, 34]. The AdaBoost algorithm chooses a good set of weak classifiers in rounds. On each round, it chooses the optimal classifier, which consists of feature values and the threshold, so that some misclassified data in the previous round would be correctly classified.
About this article
Cite this article
Nomura, Y., Hanaoka, S., Nakao, T. et al. Performance changes due to differences in training data for cerebral aneurysm detection in head MR angiography images. Jpn J Radiol 39, 1039–1048 (2021). https://doi.org/10.1007/s11604-021-01153-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-021-01153-1