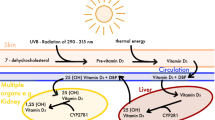
Abstract
Summary
Patients with type 2 diabetes mellitus have a higher risk of dental and/or orthopaedic implant failure. However, the mechanism behind this phenomenon is unclear, and animal studies may prove useful in shedding light on the processes involved. This review considers the available literature on rat models of diabetes and titanium implantation.
Introduction
The process of osseointegration whereby direct contact is achieved between bone and an implant surface depends on healthy bone metabolism. Collective evidence suggests that hyperglycaemia adversely affects bone turnover and the quality of the organic matrix resulting in an overall deterioration in the quality, resilience and structure of the bone tissue. This in turn results in compromised osseointegration in patients receiving dental and orthopaedic implants. The incidence of diabetes mellitus (DM), which is a chronic metabolic disorder resulting in hyperglycaemia, is rising. Of particular significance is the rising incidence of adult onset type 2 diabetes mellitus (T2DM) in an ageing population. Understanding the effects of hyperglycaemia on osseointegration will enable clinicians to manage health outcomes for patients receiving implants. Much of our understanding of how hyperglycaemia affects osseointegration comes from animal studies.
Methods
In this review, we critically analyse the current animal studies.
Results
Our review has found that most studies used a type 1 diabetes mellitus (T1DM) rodent model and looked at a young male population of rodents. The pathophysiology of T1DM is however very different to that of T2DM and is not representative of T2DM, the incidence of which is rising in the ageing adult population. Genetically modified rats have been used to model T2DM, but none of these studies have included female rats and the metabolic changes in bone for some of these models used are not adequately characterized.
Conclusions
Therefore, the review suggests that the study population needs to be broadened to include both T1DM and T2DM models, older rats as well as young rats, and importantly animals from both sexes to reflect more accurately clinical practice.
Similar content being viewed by others
References
Association AD (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 36(Supplement 1):S67–S74
International Diabetes Federation. IDF Diabetes, 7th ed. Brussels, Belgium: International Diabetes Federation, 2015.www.diabetesatlas.org.
Branemark PI, Adell R, Breine U, Hansson BO, Lindstrom J, Ohlsson A (1969) Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand J Plast Reconstr Surg 3(2):81–100
Marx RE, Garg AK (1998) Bone structure, metabolism, and physiology: its impact on dental implantology. Implant Dent 7(4):267–276
Bryant SR, Zarb GA (1998) Osseointegration of oral implants in older and younger adults. Int J Oral Maxillofac Implants 13(4):492–499
Chrcanovic B, Albrektsson T, Wennerberg A (2014) Diabetes and oral implant failure a systematic review. J Dent Res 93(9):859–867
King AJF (2012) The use of animal models in diabetes research. Br J Pharmacol 166(3):877–894
Bell RH, Hye RJ (1983) Animal models of diabetes mellitus: physiology and pathology. J Surg Res 35(5):433–460
Srinivasan K, Ramarao P (2007) Animal models in type 2 diabetes research: an overview. Indian J Med Res 125(3):451
Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, et al. (2009) Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J Clin Endocrinol Metab 94(1):45–49
Krakauer JC, McKenna MJ, Buderer NF, Rao DS, Whitehouse FW, Parfitt AM (1995) Bone loss and bone turnover in diabetes. Diabetes 44(7):775–782
Pedrazzoni M, Ciotti G, Pioli G, Girasole G, Davoli L, Palummeri E, et al. (1989) Osteocalcin levels in diabetic subjects. Calcif Tissue Int 45(6):331–336
Shu A, Yin M, Stein E, Cremers S, Dworakowski E, Ives R, et al. (2012) Bone structure and turnover in type 2 diabetes mellitus. Osteoporos Int 23(2):635–641
Achemlal L, Tellal S, Rkiouak F (2005) Bone metabolism in male patients with type 2 diabetes. Clin Rheumatol 24:493–496
Knudsen ST, Foss CH, Poulsen PL, Andersen NH, Mogensen CE, Rasmussen LM (2003) Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. European journal of endocrinology / European Federation of Endocrine Societies 149(1):39–42
Vashishth D (2007) The role of the collagen matrix in skeletal fragility. Current osteoporosis reports 5(2):62–66
Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, et al. (2006) Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 38(3):300–309
Kume S, Kato S (2005) Yamagishi Si, Inagaki Y, Ueda S, Arima N, et al. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res 20(9):1647–1658
Miyata T, Notoya K, Yoshida K, Horie K, Maeda K, Kurokawa K, et al. (1997) Advanced glycation end products enhance osteoclast-induced bone resorption in cultured mouse unfractionated bone cells and in rats implanted subcutaneously with devitalized bone particles. J Am Soc Nephrol 8(2):260–270
Levinger I, Seeman E, Jerums G, McConell GK, Rybchyn MS, Cassar S, et al. (2016) Glucose-loading reduces bone remodeling in women and osteoblast function in vitro. Physiological reports 4(3):e12700
Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, et al. (2010) High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. The Journal of Clinical Endocrinology & Metabolism 95(11):5045–5055
Hasegawa H, Ozawa S, Hashimoto K, Takeichi T, Ogawa T (2008) Type 2 diabetes impairs implant osseointegration capacity in rats. Int J Oral Maxillofac Implants 23:237–246
Berglundh T, Abrahamsson I, Lang NP, Lindhe J (2003) De novo alveolar bone formation adjacent to endosseous implants. Clin Oral Implants Res 14(3):251–262
Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229.
Nevins ML, Karimbux NY, Weber HP, Giannobile WV, Fiorellini JP (1998) Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants 13(5):620–629
McCracken MS, Aponte-Wesson R, Chavali R, Lemons JE (2006) Bone associated with implants in diabetic and insulin-treated rats. Clin Oral Implants Res 17(5):495–500
Kwon PT, Rahman SS, Kim DM, Kopman JA, Karimbux NY, Fiorellini JP (2005) Maintenance of osseointegration utilizing insulin therapy in a diabetic rat model. J Periodontol 76(4):621–626
Siqueira JT, Cavalher-Machado SC, Arana-Chavez VE, Sannomiya P (2003) Bone formation around titanium implants in the rat tibia: role of insulin. Implant Dent 12(3):242–251
Yamamoto K, Yamamoto S, Iizuka T, Obata T, Imai Y, Yanagawa A, et al. (2006) Effect of duration of hyperglycemia on osseointegration around titanium implants. Journal of Oral Biosciences 48(1):62–73
Kuchler U, Spilka T, Baron K, Tangl S, Watzek G, Gruber R (2011) Intermittent parathyroid hormone fails to stimulate osseointegration in diabetic rats. Clin Oral Implants Res 22(5):518–523
Molon RS, Morais-Camilo JAND, Verzola MHA, Faeda RS, Pepato MT, Marcantonio E (2013) Impact of diabetes mellitus and metabolic control on bone healing around osseointegrated implants: removal torque and histomorphometric analysis in rats. Clin Oral Implants Res 24(7):831–837
Retzepi M, Lewis MP, Donos N (2010) Effect of diabetes and metabolic control on de novo bone formation following guided bone regeneration. Clin Oral Implants Res 21(1):71–79
Margonar RDDSMS, Sakakura CEDDSMS, Holzhausen MDDSMS, Pepato MTP, Candia Alba RJDDS, Marcantonio EJDDSMSP (2003) The influence of diabetes mellitus and insulin therapy on biomechanical retention around dental implants: a study in rabbits. Implant Dent 12(4):333–339
Donos N, Dereka X, Mardas N (2015) Experimental models for guided bone regeneration in healthy and medically compromised conditions. Periodontol 68(1):99–121
Hough S, Avioli LV, Bergfeld MA, Fallon MD, Slatopolsky E, Teitelbaum SL (1981) Correction of abnormal bone and mineral metabolism in chronic streptozotocin-induced diabetes mellitus in the rat by insulin therapy. Endocrinology 108(6):2228–2234
Nevins ML, Karimbux NY (1998) Wound healing around endosseous implants in experimental diabetes. Int J Oral Maxillofac Implants 13(5):620–629
Ajami E, Mahno E, Mendes V, Bell S, Moineddin R, Davies J (2014) Bone healing and the effect of implant surface topography on osteoconduction in hyperglycemia. Acta Biomater 10(1):394–405
de Morais JA, Trindade-Suedam IK, Pepato MT, Marcantonio E Jr, Wenzel A, Scaf G (2009) Effect of diabetes mellitus and insulin therapy on bone density around osseointegrated dental implants: a digital subtraction radiography study in rats. Clin Oral Implants Res 20(8):796–801
Kotsovilis S, Karoussis IK, Fourmousis I (2006) A comprehensive and critical review of dental implant placement in diabetic animals and patients. Clin Oral Implants Res 17(5):587–599
Glösel B, Kuchler U, Watzek G, Gruber R (2010) Review of dental implant rat research models simulating osteoporosis or diabetes. International Journal of Oral & Maxillofacial Implants 25(3):516–524
Casap N, Nimri S, Ziv E, Sela J, Samuni Y (2008) Type 2 diabetes has minimal effect on osseointegration of titanium implants in Psammomys obesus. Clin Oral Implants Res 19(5):458–464
Wang F, Song Y-L, Li D-H, Li C-x, Wang Y, Zhang N, et al. (2010) Type 2 diabetes mellitus impairs bone healing of dental implants in GK rats. Diabetes Res Clin Pract 88(1):e7–e9
Wang F, Song YL, Li CX, Li DH, Zhang HP, Ma AJ, et al. (2010) Sustained release of insulin-like growth factor-1 from poly(lactide-co-glycolide) microspheres improves osseointegration of dental implants in type 2 diabetic rats. Eur J Pharmacol 640(1–3):226–232
Wang B, Song Y, Wang F, Li D, Zhang H, Ma A, et al. (2011) Effects of local infiltration of insulin around titanium implants in diabetic rats. Br J Oral Maxillofac Surg 49(3):225–229
Yu M, Zhou W, Song Y, Yu F, Li D, Na S, et al. (2011) Development of mesenchymal stem cell-implant complexes by cultured cells sheet enhances osseointegration in type 2 diabetic rat model. Bone 49(3):387–394
Zou GK, Song YL, Zhou W, Yu M, Liang LH, Sun DC, et al. (2012) Effects of local delivery of bFGF from PLGA microspheres on osseointegration around implants in diabetic rats. Oral surgery, oral medicine, oral pathology and oral radiology 114(3):284–289
Sugita Y, Honda Y, Kato I, Kubo K, Maeda H, Ogawa T (2014) Role of photofunctionalization in mitigating impaired osseointegration associated with type 2 diabetes in rats. Int J Oral Maxillofac Implants 29(6):1293–1300
Liu Z, Zhou W, Tangl S, Liu S, Xu X, Rausch-Fan X (2015) Potential mechanism for osseointegration of dental implants in Zucker diabetic fatty rats. Br J Oral Maxillofac Surg 53(8):748–753
Zhou W, Liu Z, Yao J, Chi F, Dong K, Yue X, et al. (2015) The effects of exenatide microsphere on serum BGP and ALP levels in ZDF rats after implantation. Clin Implant Dent Relat Res 17(4):765–770
Hashiguchi C, Kawamoto S, Kasai T, Nishi Y, Nagaoka E (2014) Influence of an antidiabetic drug on biomechanical and histological parameters around implants in type 2 diabetic rats. Implant Dent 23(3):264–269
Rees DA, Alcolado JC (2005) Animal models of diabetes mellitus. Diabetic medicine: a journal of the British Diabetic Association 22(4):359–370
Hughes P, Tanner J (1970) The assessment of skeletal maturity in the growing rat. J Anat 106(Pt 2):371
Kawano K, Hirashima T, Mori S, Natori T (1994) OLETF (Otsuka Long-Evans Tokushima Fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract 24(Suppl):S317–S320
Fukaya N, Mochizuki K, Shimada M, Goda T (2009) The α-glucosidase inhibitor miglitol decreases glucose fluctuations and gene expression of inflammatory cytokines induced by hyperglycemia in peripheral leukocytes. Nutrition 25(6):657–667
Yang SJ, Choi JM, Kim L, Park SE, Rhee EJ, Lee WY, et al. (2014) Nicotinamide improves glucose metabolism and affects the hepatic NAD-sirtuin pathway in a rodent model of obesity and type 2 diabetes. J Nutr Biochem 25(1):66–72
Kim JY, Lee SK, Jo KJ, Song DY, Lim DM, Park KY, et al. (2013) Exendin-4 increases bone mineral density in type 2 diabetic OLETF rats potentially through the down-regulation of SOST/sclerostin in osteocytes. Life Sci 92(10):533–540
Phillips MS, Liu Q, Hammond HA, Dugan V, Hey PJ, Caskey CJ, et al. (1996) Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet 13(1):18–19
Peterson RG, Shaw WN, Neel M-A, Little LA, Eichberg J (1990) Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR J 32(3):16–19
Srinivasan K, Ramarao P (2007) Animal models in type 2 diabetes research: an overview. Indian J Med Res 125(3):451–472
Pick A, Clark J, Kubstrup C, Levisetti M, Pugh W, Bonner-Weir S, et al. (1998) Role of apoptosis in failure of beta-cell mass compensation for insulin resistance and beta-cell defects in the male Zucker diabetic fatty rat. Diabetes 47(3):358–364
Fajardo RJ, Karim L, Calley VI, Bouxsein ML (2014) A review of rodent models of type 2 diabetic skeletal fragility. J Bone Miner Res 29(5):1025–1040
GOTO Y, Kakizaki M, Masaki N (1976) Production of spontaneous diabetic rats by repetition of selective breeding. Tohoku J Exp Med 119(1):85–90
Abdel-Halim SM, Guenifi A, Luthman H, Grill V, Efendic S, Östenson C-G (1994) Impact of diabetic inheritance on glucose tolerance and insulin secretion in spontaneously diabetic GK-Wistar rats. Diabetes 43(2):281–288
Cao Y, DuBois DC, Sun H, Almon RR, Jusko WJ (2011) Modeling diabetes disease progression and salsalate intervention in Goto-Kakizaki rats. J Pharmacol Exp Ther 339(3):896–904
Portha B, Giroix M, Serradas P, Gangnerau M, Movassat J, Rajas F, et al. (2001) Beta-cell function and viability in the spontaneously diabetic GK rat: information from the GK/Par colony. Diabetes 50(suppl 1):S89
Zhang L, Liu Y, Wang D, Zhao X, Qiu Z, Ji H, et al. (2009) Bone biomechanical and histomorphometrical investment in type 2 diabetic Goto-Kakizaki rats. Acta Diabetol 46(2):119–126
Scherzer P, Katalan S, Got G, Pizov G, Londono I, Gal-Moscovici A, et al. (2011) Psammomys obesus, a particularly important animal model for the study of the human diabetic nephropathy. Anatomy & cell biology 44(3):176–185
Walder KR, Fahey RP, Morton GJ, Zimmet PZ, Collier GR (2000) Characterization of obesity phenotypes in Psammomys obesus (Israeli sand rats). Journal of Diabetes Research 1(3):177–184
Amir G, Adler J, Menczel J (1991) Histomorphometric analysis of weight bearing bones of diabetic and non-diabetic sand rats (Psammomys obesus). Diabetes research (Edinburgh, Scotland) 17(3):135–137
Srinivasan K, Patole PS, Kaul CL, Ramarao P (2004) Reversal of glucose intolerance by by pioglitazone in high fat diet-fed rats. Methods Find Exp Clin Pharmacol 26(5):327–333
Storlien L, James D, Burleigh K, Chisholm D, Kraegen E (1986) Fat feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. American Journal of Physiology-Endocrinology And Metabolism 251(5):E576–EE83
Lavet C, Martin A, Linossier MT, Bossche AV, Laroche N, Thomas M, et al. (2016) Fat and sucrose intake induces obesity-related bone metabolism disturbances: kinetic and reversibility studies in growing and adult rats. J Bone Miner Res 31(1):98–115
Albrektsson T, Wennerberg A (2004) Oral implant surfaces: part 1—review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont 17(5):536–543
Klein GL (2014) Insulin and bone: recent developments. World J Diabetes 5(1):14–16
Leslie WD, Rubin MR, Schwartz AV, Kanis JA (2012) Type 2 diabetes and bone. J Bone Miner Res Off J Am Soc Bone Miner Res 27(11):2231–2237
McCullough LD, de Vries GJ, Miller VM, Becker JB, Sandberg K, McCarthy MM (2014) NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol Sex Differ 5:15
Compston JE (2001) Sex steroids and bone. Physiol Rev 81(1):419–447
Gale EA, Gillespie KM (2001) Diabetes and gender. Diabetologia 44(1):3–15
Huxley R, Barzi F, Woodward M (2006) Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ: British Medical Journal 332(7533):73–78
French D, Larjava H, Ofec R (2015) Retrospective cohort study of 4591 Straumann implants in private practice setting, with up to 10-year follow-up. Part 1: multivariate survival analysis. Clin Oral Implants Res 26(11):1345–1354
Chrcanovic BR, Kisch J, Albrektsson T, Wennerberg A. (2016) Factors influencing early dental implant failures. J Dent Res
Acknowledgments
A/Prof. Levinger was supported by Future Leader Fellowship (ID 100040) from the National Heart Foundation of Australia, and Dr. Brennan-Speranza was supported by an NHMRC Early Career Research Fellowship (ID 1013295).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
King, S., Klineberg, I., Levinger, I. et al. The effect of hyperglycaemia on osseointegration: a review of animal models of diabetes mellitus and titanium implant placement. Arch Osteoporos 11, 29 (2016). https://doi.org/10.1007/s11657-016-0284-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-016-0284-1