Abstract
Summary
This review provides a critical analysis of currently available approaches to increase bone mass, structure and strength through drug therapy and of possible direct intra-osseous interventions for the management of patients at imminent risk of hip fracture.
Purpose
Osteoporotic hip fractures represent a particularly high burden in morbidity-, mortality- and health care-related costs. There are challenges and unmet needs in the early prevention of hip fractures, opening the perspective of new developments for the management of osteoporotic patients at imminent and/or at very high risk of hip fracture. Amongst them, preventive surgical intervention needs to be considered.
Methods
A European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO)/International Osteoporosis Foundation (IOF) working group reviewed the presently available intervention modalities including preventive surgical options for hip fragility. This paper represents a summary of the discussions.
Results
Prevention of hip fracture is currently based on regular physical activity; prevention of falls; correction of nutritional deficiencies, including vitamin D repletion; and pharmacological intervention. However, efficacy of these various measures to reduce hip fractures is at most 50% and may need months or years before becoming effective. To face the challenges of early prevention of hip fractures for osteoporotic patients at imminent and/or at very high risk of hip fracture, preventive surgical intervention needs further investigation.
Conclusion
Preventive surgical intervention needs to be appraised for osteoporotic patients at imminent and/or at very high risk of hip fracture.
Similar content being viewed by others
Introduction
Osteoporosis is a silent, asymptomatic disease affecting millions of older people of all ethnicities. The disease is disproportionately observed in women, and it is estimated that 200 million women worldwide have osteoporosis [1]. The prevalence of osteoporosis in Europe in 2010 was estimated at 27.6 million, of which 22 million were women [1, 2]. Osteoporosis is associated to a high risk of low-energy fractures of the hip, spine, proximal humerus or forearm. This risk significantly increases with age, and approximately 90% of the fractures are sustained in patients aged 60 years and older [3].
In the year 2000, there were an estimated 8.9 million new osteoporotic fractures worldwide, comprising 1.6 million hip fractures, 1.7 million forearm fractures and 1.4 million vertebral fractures. For these fractures, the female-to-male ratio was 1.6 [4]. In the Western world, the lifetime risk of any fracture in women over the age of 50 years is in the order of 40–51% and for hip fracture is 18–23% [5, 6]. Hip fracture is the most severe type of fracture in osteoporotic patients. Its incidence is highly variable from country to country (<100 to >400 per 100,000 habitants), as well as is the age-related exponential increase with age [7]. By 2050, the worldwide annual number of hip fractures is expected to reach 4.5 to 6.3 million, reflecting the continuous ageing of the population [8, 9].
Osteoporotic hip fractures represent a particularly high burden in economic costs as compared to other fracture sites [2]. Hip fracture commonly leads to long-term physical disability, reducing quality of life and impairing the capacity to live independently and perform daily activities [10]. The cascade of consequences associated with hip fractures also includes depression (that may result in slowed recovery), health co-morbidities and increased risk of death [11–16].
Following a hip fracture, all-cause mortality rates are higher within the first 3 months (5.75-fold higher in women and 7.95-fold higher in men compared to controls) [17]. In observational studies across the USA and Europe, 8–36% of patients died within the first year [18–21], and the 5-year mortality risk in this population was evaluated at 64% [22]. The 1-year morbidity rates after a hip fracture are higher in elderly patients suffering from depression, delirium or dementia, highlighting the need for personalised care [23]. It is difficult to determine the proportion of deaths that are causally related to the hip fracture rather than due to morbidity, but indirect evidence from the pattern of mortality with time suggests that approximately 30% of deaths are a direct result of the fracture event [24].
The burden of osteoporosis as evaluated by disability-adjusted life years (DALYs) in Europe and the Americas accounts for 2 million DALYs annually, which is more than those explained by hypertensive heart disease [2]. In the USA, in 2005, osteoporosis-related hip fractures in men and women over the age of 50 cost nearly $12.2 billion [25]. The economic burden of incident and prior fragility fractures in the European Union was estimated at € 37 billion in 2010. Incident fractures represented 66% of this cost, long-term fracture care 29% and pharmacological prevention 5%. These costs are expected to increase by 25% in 2025 [2].
Several tools have been developed to detect patients at increased risk of fracture. The FRAX assessment tool is widely used to evaluate the fracture risk in osteoporotic patients [26]. This tool based on simple questions assessing bone mineral density (BMD)-independent risk factors provides 10-year probability of experiencing major osteoporotic fractures or specifically hip fracture. BMD at the femoral neck can optionally be entered to enhance fracture prediction.
Second hip fractures tend to occur early following an initial hip fracture. Annual incidence for a second hip fractures ranges from 2 to 10% with a mean interval between first and second hip fracture of approximately 2 years [27–29]. In hip fracture patients, the cumulative incidences for second hip fracture range between 2.4 and 5.1% within the first year, 5.1 and 8.1% within the first 2 years, 8.0 and 8.2% at 5 years and 8.6% within the first 8 years [28, 30–32]. A retrospective study of 241 hip fractures also reported that 11% of the patients had previously experienced a contralateral hip fracture on average 5.6 years before [33]. This risk of recurrent hip fracture persists for at least 10 years amongst hip fracture survivors, with a reported risk of 8% of a second fracture within 10 years [34, 35].
In this context, the benefits of coordinator-based systems to ensure appropriate management of patients following a fracture are well established [36–38]. The International Osteoporosis Foundation (IOF) has launched a global campaign (‘Capture the fracture ™ ’) to promote this approach for the prevention of a second fracture [36]. This initiative aims to set internationally endorsed standards for best practice by facilitating the implementation of fracture liaison services (FLSs) involving best practice frameworks, multidisciplinary models and FLS questionnaires [36–38]. Despite the major burden associated with osteoporosis and fragility fracture and the antifracture efficacy of the available therapies, there is a marked treatment gap in osteoporosis, i.e. a significant proportion of patients at high fracture risk or those who have sustained a fragility fracture are not being treated [1, 2]. In Europe, the treatment gap for osteoporosis ranges from 63 to 80% according to country and gender [1]. Only moderate usage of treatments like calcium and vitamin D (8–62%, median 18%) postfracture is seen. Bisphosphonate usage is lower (0.5–38%) [39]. In patients who sustain a fragility fracture, it was reported that fewer than 20% of individuals receive therapies (about 5% in the case of a first hip fracture) [40]. More recent data indicate that in Europe and the USA, less than 25 and 20%, respectively, of patients with a hip fracture receive osteoporosis medication, with less than 15% receiving bisphosphonates [41].
These considerations highlight the need for appropriate management options, particularly in very high-risk osteoporotic patients. This review provides a critical analysis of currently available approaches to increase bone mass, structure and strength through drug therapy and of possible direct intra-osseous interventions for the management of patients at imminent risk of hip fracture.
Methods
Relevant articles and reviews were identified through PubMed/MEDLINE by working group members. Other items were selected from the reference lists of related articles and the presentations during the working group meeting.
Pathogenesis of osteoporotic fracture
A bone fracture occurs when the externally applied load exceeds its intrinsic load-bearing capacity. Thus, the resulting bone deformation initiates tissue damage, which results in a major crack through the bone structure (Fig. 1). Recent data from a prospective study where hip strength was estimated by finite element analyses (FEAs) actually demonstrates that virtually, all hip fractures occur in subjects whose hip strength is below 3000 N [42]. Areal bone mineral density (aBMD), as measured by dual-energy X-ray absorptiometry (DXA), is the most commonly used surrogate of bone strength [42–45]. aBMD accounts for 60 to 80% of bone strength variance. However, bone strength also depends on bone geometry, microstructure and material properties [46–48]. It is therefore important to recognise that the annual percentage loss of hip strength exceeds the percentage loss of areal bone mineral density and this difference increases exponentially with age, emphasizing the importance of structural changes as a determinant of bone fragility [49].
With the menopause and then ageing, early trabecular thinning and loss of connectivity occur, leading to a weakening of predominantly cancellous bone parts such as the vertebrae and ultradistal radius. At the latter site, for instance, a 20% loss of trabecular number or thickness have been estimated to cause a 10% loss of strength [50]. However, even at the distal radius, the thin cortical shell (typically less than 1 mm) carries more than 50% of the load [51], so that a 20% loss of cortical bone volume at that site would cause a 37% loss of strength [50]. Hence, cortical thickness is a major contributor to bone strength; however, its importance varies according to the bone site and the mechanism of the fracture [52, 53]. Although there is evidence that part of the axial load carried by the cortical shell in the human vertebra increases with age [54], the overall stiffness contribution of the cortical shell is almost balanced with that of the trabecular component alone [55]. Cortical porosity increases with age and represents a major contribution of the decrease in bone material stiffness and toughness [56]. Hence, with ageing, the cortical bone becomes substantially thinner, and more porous, leading to loss of elasticity and increased fracture risk in a region, which undergoes compression during a fall [57, 58]. At the hip, cortical thinning occurs asymmetrically with ageing, so that the percentage of bone loss is greater in the thinner superior cortex than in the thicker inferior portion [59].
There is a shifting opinion regarding the origin of a hip fracture, particularly with respect to the initiation of failure in trabecular vs. cortical bone, and the importance of the trabecular network to contribute to hip strength. In the proximal femur, a study suggested that removing the trabecular core of the hip reduces by only 7% the all-hip strength in compression, which suggests a major contribution of cortical bone [52]. However, this study has been heavily criticised because the fracture was produced in stance loading and not in a sideway fall loading. In the latter case scenario, fracture mechanisms as identified in cadaver bone appear to involve compression failure on the supero-lateral side (a region with little cortical bone) and tension failure on the medial side (rich in cortical bone) [60]. But, trabecular bone has the ability to absorb some excess energy. In weakened, osteoporotic bones, increasing trabecular strength provides improved structural redundancy, thereby increasing the threshold for fracture initiation [61]. Cortical bone appears only to be engaged in the failure process when there is insufficient trabecular strength in the trochanteric and subcapital femoral neck regions to withstand the fall loading [61]. Once the cortex gets involved, tissue-level failure appears in both bone compartments, with equal contribution.
Therefore, in designing new approaches to restore bone strength of the hip, both compartments should be considered for reinforcement.
Current management options
Non-pharmacological options
Preventive measures include lifestyle interventions, fall prevention and possibly the use of hip protectors (Fig. 2). Hip pads are an example of such an approach, as they aim to reduce to the amount of mechanical energy transmitted to the hip following a fall, with the hope that this will reduce the likelihood of fracture. The effectiveness of this intervention is controversial and also limited by poor compliance and acceptance [63–65].
The fracture prevention triangle [62]. Adapted from Cryer C, Patel S. Falls, fragility and fractures. National service framework for older people. The case for and strategies to implement a joint health improvement and modernisation plan for falls and osteoporosis. London: Alliance for Better Bone Health, 2001
Non-pharmacological interventions include dietary modification, stopping smoking, avoiding excessive alcohol intake, regular weight bearing, balance restoring and muscle-strengthening exercises [26, 66, 67]. Increasing physical activity, in combination of a nutritional intervention enriched in calcium, proteins and vitamin D, has been associated with increases in BMD in postmenopausal women and improvement of muscle strength [67–71]. Whilst adopting these measures may have beneficial effects on bone health, evidence that they prevent fractures is lacking [72]. Guidelines also stress the importance of taking measures to prevent falls. However, although fall prevention programmes have been shown to reduce the frequency of falls, the majority of studies in elderly subjects have failed to show fracture reduction [73, 74].
Pharmacological treatments
Current pharmaceutical therapies for osteoporosis have been shown to increase femoral neck and/or total hip aBMD and, for some treatments, to reduce hip fracture risk. Across all placebo-controlled trials involving all sorts of osteoporosis drugs, hip BMD gain explains near to 60% of hip fracture reduction [75]. However, comparison between medications is difficult because of largely different fracture risk in the studied populations.
Alendronate, risedronate, zoledronic acid and denosumab have all been shown to significantly decrease the risk of hip fracture by approximately 40–50% in preplanned intention to treat analyses [76–81]. This percentage reduction can be considered as a benchmark against which to compare new drug efficacy or interventions.
Alendronate
A meta-analysis of 6 randomised controlled trials in 6804 women with T-scores ≤−2.5 treated with alendronate demonstrated a 55% risk reduction in hip fractures [81].
Risedronate
The HIP study evaluated 9331 women into two groups; one group had diagnosed osteoporosis, and the other had osteopenia but were considered to be at high risk of hip fracture, since being older than 80 years and presenting non-skeletal risk factors including poor gait and propensity to falls. The incidence of hip fracture amongst all women assigned to receive risedronate was 2.8% compared to 3.9% amongst placebo-treated women (corresponding to a 28% reduction). In the women with osteoporosis, the incidence was 1.9% in risedronate treated vs. 3.2% in placebo treated (40% reduction) [80]. However, the protective effects for hip fracture with risedronate were not significant in women aged 80 years and older, who were not selected on the base of BMD but purportedly on their risk of falling, as compared to younger patients [80].
Zoledronic acid
The Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) trial enrolled 7765 postmenopausal women with osteoporosis and demonstrated a 41% reduction in hip fracture rates following 3 years of zoledronic acid treatment when compared to placebo [76].
Furthermore, the risk of a second hip fracture was assessed; the 30% reduction in the risk of a second hip fracture did not reach the level of statistical significance [82].
The lack of evidence for hip fracture reduction in women aged 75 years and older in the two HORIZON studies, as evidenced by a pooled analysis, might raise the point that recurrent falls are the most prominent mechanism for hip fracture in this population [83]. Alternatively, that the level of hip bone fragility at that age cannot easily be reversed in just few years of pharmacological therapy.
Denosumab
The FREEDOM trial evaluated 7808 patients who received subcutaneous injections of either denosumab or placebo once every 6 months for 3 years. Denosumab treatment resulted in a 40% reduction of the incidence of hip fractures [78], and increased cortical thickness at the hip and femur [84], as well as improving determinants of structure using FEA and strength [85]. Moreover, in a preplanned subgroup analysis in high-risk patients, hip fracture risk reduction in 75+ years old reached 62%.
Teriparatide
In a trial of 1637 postmenopausal women with osteoporosis, 20 and 40 μg of teriparatide daily subcutaneously were shown to reduce the incidence of new non-vertebral fragility fractures (NVFs) by 35 to 53% [86]. Hip fractures were not reduced, but there were less than 1% of women with a hip fracture in this 18-month trial. In a review, Eriksen et al. [87] suggest potential benefits of teriparatide on hip fracture risk.
In a study conducted on over 4000 patients (the Direct Assessment of non-vertebral fractures in Community Experience (DANCE) study), the incidence rate of NVF was beyond 6 months of treatment [88].
In the European Forsteo Observational Study (EFOS), there was a 47% decrease in the odds of fracture in the last 6-month period of the 18-month trial. The NVF rates were significantly decreased between the first and the last 6-month period of treatment [89].
Strontium ranelate
The Treatment of Peripheral Osteoporosis (TROPOS) study evaluated the effects of strontium ranelate in 5091 postmenopausal women with osteoporosis. The trial demonstrated a 36% risk reduction for hip fracture in a post hoc analysis subgroup (>74 years of age and osteoporosis) over 3 years [90].
Odanacatib (investigational drug)
In a phase III long-term trial conducted on more than 16,000 postmenopausal women with prior vertebral fracture, odanacatib, an investigational cathepsin K inhibitor, showed a 47% relative reduction in hip fractures [91].
Abaloparatide (investigational drug)
Abaloparatide is a 34 amino acid peptide with 71% homology with PTHrP (1–34) and 41% homology with PTH (1–34) that has been developed for subcutaneous delivery in the treatment of osteoporosis. A phase III, randomised controlled trial, “ACTIVE,” in a cohort of ambulatory postmenopausal female patients with osteoporosis evaluated the effect of abaloparatide-SC (80 μg daily) on fracture risk. The number of hip fractures was too small (n = 3) to allow any analysis, but non-vertebral fractures were significantly reduced by 43% [92].
Number needed to treat
The number of patients needed to treat (NNT) over a period of 3 years to prevent one hip fracture varies between 48 for strontium ranelate and 200 for denosumab. NNT amounts to 91 for alendronate, risedronate and zoledronic acid [93]. The NNT does not allow one to make any comparison between the various agents since it is mainly determined by the absolute risk, i.e. osteoporosis severity and clinical risk factors, in the placebo group. Nevertheless it indicates how many women at high risk of hip fractures—as included in those trials—would need to be exposed to some sort of invasive procedure (see below) to eventually prevent a hip fracture, in the event that a local form of therapy was to be developed.
Current challenges
Poor adherence
Current treatments reduce hip fracture risk by up to 50% [94]. In the ‘real-world’ setting, this residual risk is amplified by poor adherence [95].
Whilst some regimens are convenient, particularly the injectable 6-month and 1-year forms, patient compliance to osteoporosis medications is poor and often below 50% after 1 year. Up to 60% of patients who take a once-weekly bisphosphonate and nearly 80% of those who take a once-daily bisphosphonate discontinue treatment within a year [96].
Lack of adherence may arise from side effects from the medications (e.g. gastrointestinal irritation) or fear of rare adverse events (e.g. atypical femoral fractures, osteonecrosis of the jaw) [97–99]. It could lead to a marked decrease in antifracture efficacy of the prescribed treatments. Under-prescription of efficacious drugs by caregivers despite markedly elevated fracture risk should also be mentioned.
Onset of fracture risk reduction
Several studies have suggested that there is a lag time between the onset of treatment with bisphosphonates and fracture risk reduction. For example, for zoledronic acid, after the first hip fracture, the 30% reduction in the second hip fracture was evident after 16 months of treatment [76, 78, 100, 101]. For denosumab, the incidence curves appeared to diverge by about 9 months [78]. The study of the onset of effect is problematic due to the low number of outcome events but, if true, has implications for patient care. Furthermore, the onset of action of different medications in different study populations can barely be compared, since a higher risk of fracture in the population under study is associated with an earlier achievement of statistical significance.
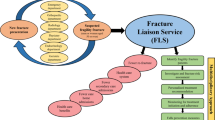
These considerations may indicate a need for early efficacious therapeutic options for those patients at imminent and very high risk of hip fracture. This would be particularly relevant following a first hip fracture. Indeed, the relative risk of re-fracture increases rapidly following a fracture and is maximal in the first year. To address these unmet needs, a specific subtype of patients that would benefit from additional approaches, including surgical preventive bone enhancement, may need to be identified (Fig. 3). Nevertheless, the risk of second fracture persists 10 years after the first one; therefore, secondary prevention should continue beyond an early postfracture period [22, 27, 29].
Possible role of orthopaedic interventions to strengthen bone
In addition to systemic treatments, procedures developed for fracture immobilisation or for filling up local bone defect (e.g. bone tumours/cysts, hemangioma, avascular necrosis) have been proposed to strengthen fragile bone, particularly proximal femur, prior to fracture occurrence [102]. Techniques are prophylactic nailing, femoroplasty with cement and bone grafting with osteoconductive or osteoinductive materials (Table 1) [103]. Like for most of the systemic therapies, these approaches do not correct the microstructural alterations. Further studies are required to identify indications and to get registration.
The main target for preventive orthopaedic surgery is the frail oldest old, at markedly elevated risk of fracture, who is the most likely to benefit from fracture prevention. This type of patients is also the most susceptible to present co-morbidities and to develop presurgery and postsurgery medical complications. In addition, anaesthesia’s risks should also be integrated into a risk/benefit assessment with quality of remaining life years as the outcome.
Prophylactic nailing
To stabilise incomplete femoral atypical fractures, prophylactic nailing has been recommended [104]. Such a prophylactic nailing for highly fragile osteoporotic hips is not currently performed in clinical practice [104]. In a randomised controlled trial, contralateral hip fixation using a hydroxy-apatite-coated titanium tubular screw was evaluated on the risk of recurrent fracture [105]. Although the feasibility and safety of the procedure were confirmed, the results were not conclusive since no contralateral hip fracture occurred over the 16-month follow-up. There is a device called Y-STRUT®, which is indicated for contralateral percutaneous internal fixation of proximal femur in patients with a low-energy pertrochanteric fracture. This device is implanted during the same anaesthesia [106]. The implant consists of two interlocking peek rods linked with surgical cement (polymethylmethacrylate (PMMA)). The loadings until failure of cadaver specimens with this implant showed increased both fracture load (+18%) and energy to fracture (+32%) as compared to contralateral femur. However, periprosthetic fracture risk should be taken into consideration in a careful benefit-risk and cost-effectiveness analysis for any new local procedure aimed at preventing hip fractures [107]. Indeed, prophylactic fixation with a cephalomedullary nail was not found to be cost-effective in elderly women with hip fracture [108]. However, the case may differ in selected patients.
Bone grafting
Bone graft materials are useful in situations where a large volume defect needs to be refilled and also when the regenerative process is compromised (avascular necrosis, atrophic non-union). Autologous bone grafts from the iliac crest will not be reviewed here, as the duration of healing, the potential risks and complications associated with this technique are not compatible with the target population of elderly frail osteoporotic patients that require a rapid increase in bone strength [109–111].
Femoroplasty (cement augmentation)
Femoroplasty is a procedure in which cement, usually PMMA, is injected into an osteoporotic proximal femur. This technique has been only evaluated to date in cadaver or animals. The results showed 30–80% improvement in bone strength, the results being volume dependent (cement augmentations of 20 to 40 ml) and location dependent [112–114].
The major drawback of this procedure is the exothermic material properties of PMMA (temperature increases from +18 to +30 °C) that can cause osteonecrosis when a large volume of cement is injected [114].
As a consequence, Beckman et al. developed a procedure where an 8-mm perforation is drilled in cadaver femurs and 8 to 18 ml of cement is injected. With this approach, the increase in fracture load (peak load to failure) was of lower magnitude (+23–35%) [115]. In vitro verification of femoroplasty with CT imaging to improve the procedure in terms of cement distribution and location indicated an increase in the yield load of +33% vs. control osteoporotic femora, for a cement volume of 9.5 ± 1.7 ml [116]. This 33% improvement in yield load, thereby reaching a value of 3000 N, by cement injection could be considered as a benchmark for the development of future interventions.
A study compared femoral augmentation using steel spirals or cement-based femoroplasty [117]. Both metal implant and cement augmentation improved hip mechanical properties when the load was applied on the greater trochanter. Both procedures are simple, reproducible and minimally invasive. Some studies on femoroplasty failed however to detect changes in biomechanical properties. Comparing cement-based femoroplasty to a minimally invasive fixation using the blade of a proximal femur nail (PFNA™ Depuy Synthès), the authors concluded that limited V-shaped PMMA augmentation and PFNA blade insertion did not show any improvement in failure load nor energy to failure [118]. In contrast to Beckman et al. [115], another study concluded that 15 ml of cement was not sufficient to augment the proximal femur and to provide biomechanical advantage [119].
It is possible that femoroplasty may be associated with the occurrence of subtrochanteric fractures, fat embolism, circulatory damage and stress concentration [114, 115, 119]. The impact of femoroplasty on femur vascularisation is still unknown. Thus, clinical validation of the technique is a research priority.
Bone grafts with osteoconductive or osteoinductive materials
Some bone graft substitutes, such as calcium phosphate bone cements, resorbable polymers or allograft, can also serve as an intra-osseous scaffold for delivery of bone anabolic agents capable of stimulating bone formation and augmenting bone mass [120]. Synthetic bone grafts are mainly made of calcium-phosphate (e.g. hydroxyapatite and tricalcium phosphate), bioglass and calcium sulphate [121]. Such materials can be used as carriers for growth factors to enhance bone graft efficacy, drugs (bisphosphonates) or ions (strontium) to promote osteoblast proliferation [122–126]. Local administration of bone morphogenetic protein (rhBMP-2, rh-BMP-7) is another example of osteoanabolic substances use. However, the implant procedure may be accompanied by an intense first phase of bone resorption when a high dose of BMPs is used, which may preclude its use in osteoporosis [127, 128].
Presently, the bone graft administration field is very wide (with orthopaedics, traumatology, maxilla-facial/dental applications), with the spine being its largest application site (45% of procedures). The main drawback of bone graft substitutes is that they are usually used to fill a defect (e.g. left by the extraction of a tumour or a cyst). In osteoporosis, the administration of osteoconductive or osteoinductive materials requires fluid material to avoid injection under high pressure in the trabecular bone network of the proximal femur.
Conclusions
The prevention of hip fractures, the most devastating complication of osteoporosis, remains a challenge, considering that only a minority of high-risk patients, including patients with a recent hip fracture who carry at least a 10% early risk of contralateral fracture, are actually given osteoporosis therapy; that the efficacy of the most potent osteoporosis drugs to prevent hip fractures is at best 50% after more than 12–18 months of therapy; and that 50% or more of the patients may have discontinued osteoporosis therapy by then. Hence, complementary approaches to immediately prevent hip fractures in patients at very high risk may need to be developed, including surgical approaches.
Some new materials are currently being developed that are synthetic, resorbable, osteoconductive and osteoinductive materials, with the aim of locally strengthening weak bone structure, e.g. hip, to fill a gap in the management of elderly patients with an increased imminent risk of hip fracture. However, the acceptability of a minimal but still invasive procedure in frail patients added to the need to use clear surrogate markers for bone strength improvement, together with the identification of high risk most likely to benefit from the procedure, highlights the difficulty of clinical studies aimed at demonstrating the benefits and safety profile of such innovative devices. Intervention thresholds remain to be established in a more global context of an encompassing risk-benefit analysis. Overall, the benefits should be in line with the risks.
References
Svedbom A, Hernlund E, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos 8:137
Hernlund E, Svedbom A, Ivergard M, Compston J, Cooper C, Stenmark J, McCloskey EV, Jonsson B, Kanis JA (2013) Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos 8:136
Kim SH, Meehan JP, Blumenfeld T, Szabo RM (2012) Hip fractures in the United States: 2008 nationwide emergency department sample. Arthritis Care Res (Hoboken) 64:751–757
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17:1726–1733
Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B (2000) Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11:669–674
Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Heath H 3rd (1992) Risk of age-related fractures in patients with primary hyperparathyroidism. Arch Intern Med 152:2269–2273
Kanis JA, Oden A, McCloskey EV, Johansson H, Wahl DA, Cooper C (2012) A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 23:2239–2256
Cooper C, Campion G, Melton LJ 3rd (1992) Hip fractures in the elderly: a world-wide projection. Osteoporos Int 2:285–289
Gullberg B, Johnell O, Kanis JA (1997) World-wide projections for hip fracture. Osteoporos Int 7:407–413
Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE (1990) Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol 45:M101–M107
Alarcon T, Gonzalez-Montalvo JI, Gotor P, Madero R, Otero A (2011) Activities of daily living after hip fracture: profile and rate of recovery during 2 years of follow-up. Osteoporos Int 22:1609–1613
Borgstrom F, Zethraeus N, Johnell O et al (2006) Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 17:637–650
Cree M, Soskolne CL, Belseck E, Hornig J, McElhaney JE, Brant R, Suarez-Almazor M (2000) Mortality and institutionalization following hip fracture. J Am Geriatr Soc 48:283–288
Holmes JD, House AO (2000) Psychiatric illness in hip fracture. Age Ageing 29:537–546
Phillips AC, Upton J, Duggal NA, Carroll D, Lord JM (2013) Depression following hip fracture is associated with increased physical frailty in older adults: the role of the cortisol: dehydroepiandrosterone sulphate ratio. BMC Geriatr 13:60
Pratt LA, Brody DJ (2008) Depression in the United States household population, 2005–2006. NCHS Data Brief 1–8
Haentjens P, Magaziner J, Colon-Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, Boonen S (2010) Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 152:380–390
Abrahamsen B, Brixen K (2009) Mapping the prescriptiome to fractures in men—a national analysis of prescription history and fracture risk. Osteoporos Int 20:585–597
Congress US (1994) Office of Technology Assessment hip fracture outcomes in people age 50 and older, vol 120. OTA-BP-H, Washington,DC
LeBlanc ES, Hillier TA, Pedula KL et al (2011) Hip fracture and increased short-term but not long-term mortality in healthy older women. Arch Intern Med 171:1831–1837
Leibson CL, Tosteson AN, Gabriel SE, Ransom JE, Melton LJ (2002) Mortality, disability, and nursing home use for persons with and without hip fracture: a population-based study. J Am Geriatr Soc 50:1644–1650
Ryg J, Rejnmark L, Overgaard S, Brixen K, Vestergaard P (2009) Hip fracture patients at risk of second hip fracture: a nationwide population-based cohort study of 169,145 cases during 1977-2001. J Bone Miner Res 24:1299–1307
Piscitelli P, Metozzi A, Benvenuti E et al (2012) Connections between the outcomes of osteoporotic hip fractures and depression, delirium or dementia in elderly patients: rationale and preliminary data from the CODE study. Clin Cases Miner Bone Metab 9:40–44
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32:468–473
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 22:465–475
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57
Madhok R, Melton LJ 3rd, Atkinson EJ, O’Fallon WM, Lewallen DG (1993) Urban vs rural increase in hip fracture incidence. Age and sex of 901 cases 1980-89 in Olmsted County, USA. Acta Orthop Scand 64:543–548
Scaglione M, Fabbri L, Di Rollo F, Bianchi MG, Dell’omo D, Guido G (2013) The second hip fracture in osteoporotic patients: not only an orthopaedic matter. Clin Cases Miner Bone Metab 10:124–128
Schroder HM, Petersen KK, Erlandsen M (1993) Occurrence and incidence of the second hip fracture. Clin Orthop Relat Res 166–169
Berry SD, Samelson EJ, Hannan MT, McLean RR, Lu M, Cupples LA, Shaffer ML, Beiser AL, Kelly-Hayes M, Kiel DP (2007) Second hip fracture in older men and women: the Framingham study. Arch Intern Med 167:1971–1976
Lee YK, Ha YC, Yoon BH, Koo KH (2013) Incidence of second hip fracture and compliant use of bisphosphonate. Osteoporos Int 24:2099–2104
Lonnroos E, Kautiainen H, Karppi P, Hartikainen S, Kiviranta I, Sulkava R (2007) Incidence of second hip fractures. A population-based study. Osteoporos Int 18:1279–1285
Gaumetou E, Zilber S, Hernigou P (2011) Non-simultaneous bilateral hip fracture: epidemiologic study of 241 hip fractures. Orthop Traumatol Surg Res 97:22–27
Chapurlat RD, Bauer DC, Nevitt M, Stone K, Cummings SR (2003) Incidence and risk factors for a second hip fracture in elderly women. The study of osteoporotic fractures. Osteoporos Int 14:130–136
Sobolev B, Sheehan KJ, Kuramoto L, Guy P (2015) Risk of second hip fracture persists for years after initial trauma. Bone 75:72–76
Javaid MK, Kyer C, Mitchell PJ et al (2015) Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF capture the fracture (R) best practice framework tool. Osteoporos Int 26:2573–2578
Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C, Group IOFFW (2013) Capture the fracture: a best practice framework and global campaign to break the fragility fracture cycle. Osteoporos Int 24:2135–2152
Marsh D, Akesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, McLellan AR, Mitchell PJ, Sale JE, Wahl DA (2011) Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int 22:2051–2065
Elliot-Gibson V, Bogoch ER, Jamal SA, Beaton DE (2004) Practice patterns in the diagnosis and treatment of osteoporosis after a fragility fracture: a systematic review. Osteoporos Int 15:767–778
Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE (2000) Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med 109:326–328
Kim SC, Kim MS, Sanfelix-Gimeno G et al (2015) Use of osteoporosis medications after hospitalization for hip fracture: a cross-national study. Am J Med 128(519–526):e511
Kopperdahl DL, Aspelund T, Hoffmann PF, Sigurdsson S, Siggeirsdottir K, Harris TB, Gudnason V, Keaveny TM (2014) Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res 29:570–580
Engelke K, Libanati C, Fuerst T, Zysset P, Genant HK (2013) Advanced CT based in vivo methods for the assessment of bone density, structure, and strength. Curr Osteoporos Rep 11:246–255
Imai K (2014) Recent methods for assessing osteoporosis and fracture risk. Recent Pat Endocr Metab Immune Drug Discov 8:48–59
Rudang R, Zoulakis M, Sundh D, Brisby H, Diez-Perez A, Johansson L, Mellstrom D, Darelid A, Lorentzon M (2016) Bone material strength is associated with areal BMD but not with prevalent fractures in older women. Osteoporos Int 27:1585–1592
Ehrlich PJ, Lanyon LE (2002) Mechanical strain and bone cell function: a review. Osteoporos Int 13:688–700
Lanyon LE (1996) Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone 18:37S–43S
Whitmarsh T, Treece GM, Gee AH, Poole KE (2015) Mapping bone changes at the proximal femoral cortex of postmenopausal women in response to alendronate and teriparatide alone, combined or sequentially. J Bone Miner Res 30:1309–1318
Keaveny TM, Kopperdahl DL, Melton LJ 3rd, Hoffmann PF, Amin S, Riggs BL, Khosla S (2010) Age-dependence of femoral strength in white women and men. J Bone Miner Res 25:994–1001
Pistoia W, van Rietbergen B, Ruegsegger P (2003) Mechanical consequences of different scenarios for simulated bone atrophy and recovery in the distal radius. Bone 33:937–945
Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD (2008) Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23:392–399
Holzer G, von Skrbensky G, Holzer LA, Pichl W (2009) Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res 24:468–474
Koivumaki JE, Thevenot J, Pulkkinen P, Kuhn V, Link TM, Eckstein F, Jamsa T (2012) Cortical bone finite element models in the estimation of experimentally measured failure loads in the proximal femur. Bone 51:737–740
Christiansen BA, Kopperdahl DL, Kiel DP, Keaveny TM, Bouxsein ML (2011) Mechanical contributions of the cortical and trabecular compartments contribute to differences in age-related changes in vertebral body strength in men and women assessed by QCT-based finite element analysis. J Bone Miner Res 26:974–983
Eswaran SK, Gupta A, Keaveny TM (2007) Locations of bone tissue at high risk of initial failure during compressive loading of the human vertebral body. Bone 41:733–739
McCalden RW, McGeough JA, Barker MB, Court-Brown CM (1993) Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am 75:1193–1205
Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J (2005) Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet 366:129–135
Zebaze RM, Jones A, Welsh F, Knackstedt M, Seeman E (2005) Femoral neck shape and the spatial distribution of its mineral mass varies with its size: clinical and biomechanical implications. Bone 37:243–252
Johannesdottir F, Aspelund T, Reeve J, Poole KE, Sigurdsson S, Harris TB, Gudnason VG, Sigurdsson G (2013) Similarities and differences between sexes in regional loss of cortical and trabecular bone in the mid-femoral neck: the AGES-Reykjavik longitudinal study. J Bone Miner Res 28:2165–2176
Zani L, Erani P, Grassi L, Taddei F, Cristofolini L (2015) Strain distribution in the proximal human femur during in vitro simulated sideways fall. J Biomech 48:2130–2143
Nawathe S, Akhlaghpour H, Bouxsein ML, Keaveny TM (2014) Microstructural failure mechanisms in the human proximal femur for sideways fall loading. J Bone Miner Res 29:507–515
Cryer C, Patel S (2001) Falls, fragility and fractures. National service framework for older people. The case for and strategies to implement a joint health improvement and modernisation plan for falls and osteoporosis. Alliance for Better Bone Health, London
Cianferotti L, Fossi C, Brandi ML (2015) Hip protectors: are they worth it? Calcif Tissue Int 97:1–11
Santesso N, Carrasco-Labra A, Brignardello-Petersen R (2014) Hip protectors for preventing hip fractures in older people. Cochrane Database Syst Rev CD001255
Zimmerman S, Magaziner J, Birge SJ, Barton BA, Kronsberg SS, Kiel DP (2010) Adherence to hip protectors and implications for U.S. long-term care settings. J Am Med Dir Assoc 11:106–115
Kemmler W, Bebenek M, Kohl M, von Stengel S (2015) Exercise and fractures in postmenopausal women. Final results of the controlled Erlangen Fitness and Osteoporosis Prevention Study (EFOPS). Osteoporos Int 26:2491–2499
Rizzoli R, Branco J, Brandi ML et al (2014) Management of osteoporosis of the oldest old. Osteoporos Int 25:2507–2529
Bass SL, Naughton G, Saxon L, Iuliano-Burns S, Daly R, Briganti EM, Hume C, Nowson C (2007) Exercise and calcium combined results in a greater osteogenic effect than either factor alone: a blinded randomized placebo-controlled trial in boys. J Bone Miner Res 22:458–464
Iuliano-Burns S, Saxon L, Naughton G, Gibbons K, Bass SL (2003) Regional specificity of exercise and calcium during skeletal growth in girls: a randomized controlled trial. J Bone Miner Res 18:156–162
Kelley GA, Kelley KS, Tran ZV (2002) Exercise and lumbar spine bone mineral density in postmenopausal women: a meta-analysis of individual patient data. J Gerontol A Biol Sci Med Sci 57:M599–M604
Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW (1999) The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 9:1–12
Compston JE, Wyman A, FitzGerald G et al (2016) Increase in fracture risk following unintentional weight loss in postmenopausal women: the global longitudinal study of osteoporosis in women. J Bone Miner Res 31:1466–1472
Gillespie LD, Robertson MC, Gillespie WJ, Sherrington C, Gates S, Clemson LM, Lamb SE (2012) Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev CD007146
Silva RB, Eslick GD, Duque G (2013) Exercise for falls and fracture prevention in long term care facilities: a systematic review and meta-analysis. J Am Med Dir Assoc 14(685–689):e682
Black DM, Vittinghoff E, Eastell R, et al. (2015) Hip BMD by DXA cab reliably estimates reduction in hip risk in osteoporosis trials: a meta-regression ASBMR, abstract 1145
Black DM, Delmas PD, Eastell R et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Black DM, Reid IR, Boonen S et al (2012) The effect of 3 versus 6 years of zoledronic acid treatment of osteoporosis: a randomized extension to the HORIZON-Pivotal Fracture Trial (PFT). J Bone Miner Res 27:243–254
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Harris ST, Watts NB, Genant HK et al (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral efficacy with risedronate therapy (VERT) study group. JAMA 282:1344–1352
McClung MR, Geusens P, Miller PD et al (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip intervention program study group. N Engl J Med 344:333–340
Papapoulos SE, Quandt SA, Liberman UA, Hochberg MC, Thompson DE (2005) Meta-analysis of the efficacy of alendronate for the prevention of hip fractures in postmenopausal women. Osteoporos Int 16:468–474
Lyles KW, Colon-Emeric CS, Magaziner JS et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Boonen S, Eastell R, Su G et al (2012) Time to onset of antifracture efficacy and year-by-year persistence of effect of zoledronic acid in women with osteoporosis. J Bone Miner Res 27:1487–1493
Poole KE, Treece GM, Gee AH, Brown JP, McClung MR, Wang A, Libanati C (2015) Denosumab rapidly increases cortical bone in key locations of the femur: a 3D bone mapping study in women with osteoporosis. J Bone Miner Res 30:46–54
Keaveny TM, McClung MR, Genant HK et al (2014) Femoral and vertebral strength improvements in postmenopausal women with osteoporosis treated with denosumab. J Bone Miner Res 29:158–165
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Eriksen EF, Keaveny TM, Gallagher ER, Krege JH (2014) Literature review: the effects of teriparatide therapy at the hip in patients with osteoporosis. Bone 67:246–256
Silverman S, Miller P, Sebba A, Weitz M, Wan X, Alam J, Masica D, Taylor KA, Ruff VA, Krohn K (2013) The Direct Assessment of Nonvertebral Fractures in Community Experience (DANCE) study: 2-year nonvertebral fragility fracture results. Osteoporos Int 24:2309–2317
Langdahl BL, Rajzbaum G, Jakob F et al (2009) Reduction in fracture rate and back pain and increased quality of life in postmenopausal women treated with teriparatide: 18-month data from the European Forsteo Observational Study (EFOS). Calcif Tissue Int 85:484–493
Reginster JY, Brandi ML, Cannata-Andia J, Cooper C, Cortet B, Feron JM, Genant H, Palacios S, Ringe JD, Rizzoli R (2015) The position of strontium ranelate in today’s management of osteoporosis. Osteoporos Int 26:1667–1671
Bone HG, Dempster DW, Eisman JA et al (2015) Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the long-term odanacatib fracture trial. Osteoporos Int 26:699–712
Miller PD, Leder BZ, Hatterley G, et al. (2015) Effects of abaloparatide on vertebral and non-vertebral fracture incidence in postmenopausal women with osteoporosis—results of the phase 3 ACTIVE trial. Endocrine Reviews 36
Ringe JD, Doherty JG (2010) Absolute risk reduction in assessing treatment efficacy by number needed to treat. Rheumatol Int 30:863–869
Papapoulos S, Lippuner K, Roux C et al (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM extension study. Osteoporos Int 26:2773–2783
Kanis JA, Svedbom A, Harvey N, McCloskey EV (2014) The osteoporosis treatment gap. J Bone Miner Res 29:1926–1928
Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ (2007) Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc 82:1493–1501
Girgis CM, Seibel MJ (2011) Atypical femur fractures: a review of the evidence and its implication to clinical practice. Ther Adv Musculoskelet Dis 3:301–314
Schilcher J, Michaelsson K, Aspenberg P (2011) Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med 364:1728–1737
Woo C, Gao G, Wade S, Hochberg MC (2010) Gastrointestinal side effects in postmenopausal women using osteoporosis therapy: 1-year findings in the POSSIBLE US study. Curr Med Res Opin 26:1003–1009
Black DM, Cummings SR, Karpf DB et al (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture intervention trial research group. Lancet 348:1535–1541
Inderjeeth CA, Chan K, Kwan K, Lai M (2012) Time to onset of efficacy in fracture reduction with current anti-osteoporosis treatments. J Bone Miner Metab 30:493–503
Chiarello E, Tedesco G, Cadossi M, Capra P, Terrando S, Miti A, Giannini S (2016) Surgical prevention of femoral neck fractures in elderly osteoporotic patients. A literature review. Clin Cases Miner Bone Metab 13:42–45
Moore WR, Graves SE, Bain GI (2001) Synthetic bone graft substitutes. ANZ J Surg 71:354–361
Blum L, Cummings K, Goulet JA, Perdue AM, Mauffrey C, Hake ME (2016) Atypical femur fractures in patients receiving bisphosphonate therapy: etiology and management. Eur J Orthop Surg Traumatol 26:371–377
Giannini S, Luciani D, Chiarello E, Cadossi M, Tedesco G, Hoque M, Gnudi S (2011) Osteosynthetic improvement of osteoporotic bone: prevention surgery. Clin Cases Miner Bone Metab 8:51–54
Szpalski M, Gunzburg R, Aebi M, Delimoge C, Graf N, Eberle S, Vienney C (2015) A new approach to prevent contralateral hip fracture: evaluation of the effectiveness of a fracture preventing implant. Clin Biomech (Bristol, Avon) 30:713–719
Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A (2016) The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg 136:553–561
Faucett SC, Genuario JW, Tosteson AN, Koval KJ (2010) Is prophylactic fixation a cost-effective method to prevent a future contralateral fragility hip fracture? J Orthop Trauma 24:65–74
Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA (1996) Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res 300–309
Banwart JC, Asher MA, Hassanein RS (1995) Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976) 20:1055–1060
Seiler JG 3rd, Johnson J (2000) Iliac crest autogenous bone grafting: donor site complications. J South Orthop Assoc 9:91–97
Basafa E, Armiger RS, Kutzer MD, Belkoff SM, Mears SC, Armand M (2013) Patient-specific finite element modeling for femoral bone augmentation. Med Eng Phys 35:860–865
Basafa E, Murphy RJ, Kutzer MD, Otake Y, Armand M (2013) A particle model for prediction of cement infiltration of cancellous bone in osteoporotic bone augmentation. PLoS One 8:e67958
Heini PF, Franz T, Fankhauser C, Gasser B, Ganz R (2004) Femoroplasty-augmentation of mechanical properties in the osteoporotic proximal femur: a biomechanical investigation of PMMA reinforcement in cadaver bones. Clin Biomech (Bristol, Avon) 19:506–512
Beckmann J, Springorum R, Vettorazzi E et al (2011) Fracture prevention by femoroplasty—cement augmentation of the proximal femur. J Orthop Res 29:1753–1758
Basafa E, Murphy RJ, Otake Y, Kutzer MD, Belkoff SM, Mears SC, Armand M (2015) Subject-specific planning of femoroplasty: an experimental verification study. J Biomech 48:59–64
Springorum HR, Gebauer M, Mehrl A, Stark O, Craiovan B, Puschel K, Amling M, Grifka J, Beckmann J (2014) Fracture prevention by prophylactic femoroplasty of the proximal femur—metallic compared with cemented augmentation. J Orthop Trauma 28:403–409
Raas C, Hofmann-Fliri L, Hormann R, Schmoelz W (2016) Prophylactic augmentation of the proximal femur: an investigation of two techniques. Arch Orthop Trauma Surg 136:345–351
Sutter EG, Wall SJ, Mears SC, Belkoff SM (2010) The effect of cement placement on augmentation of the osteoporotic proximal femur. Geriatr Orthop Surg Rehabil 1:22–26
Sterling JA, Guelcher SA (2014) Biomaterial scaffolds for treating osteoporotic bone. Curr Osteoporos Rep 12:48–54
Dobelin N, Luginbuhl R, Bohner M (2010) Synthetic calcium phosphate ceramics for treatment of bone fractures. Chimia (Aarau) 64:723–729
Dimitriou R, Jones E, McGonagle D, Giannoudis PV (2011) Bone regeneration: current concepts and future directions. BMC Med 9:66
Kettenberger U, Ston J, Thein E, Procter P, Pioletti DP (2014) Does locally delivered zoledronate influence peri-implant bone formation?—spatio-temporal monitoring of bone remodeling in vivo. Biomaterials 35:9995–10006
Peter B, Zambelli PY, Guicheux J, Pioletti DP (2005) The effect of bisphosphonates and titanium particles on osteoblasts: an in vitro study. J Bone Joint Surg Br 87:1157–1163
Stadelmann VA, Gauthier O, Terrier A, Bouler JM, Pioletti DP (2008) Implants delivering bisphosphonate locally increase periprosthetic bone density in an osteoporotic sheep model. A pilot study. Eur Cell Mater 16:10–16
Stadelmann VA, Terrier A, Gauthier O, Bouler JM, Pioletti DP (2009) Prediction of bone density around orthopedic implants delivering bisphosphonate. J Biomech 42:1206–1211
Ekrol I, Hajducka C, Court-Brown C, McQueen MM (2008) A comparison of RhBMP-7 (OP-1) and autogenous graft for metaphyseal defects after osteotomy of the distal radius. Injury 39(Suppl 2):S73–S82
Laursen M, Hoy K, Hansen ES, Gelineck J, Christensen FB, Bunger CE (1999) Recombinant bone morphogenetic protein-7 as an intracorporal bone growth stimulator in unstable thoracolumbar burst fractures in humans: preliminary results. Eur Spine J 8:485–490
Acknowledgments
We are grateful to Dr. Dominique Pierroz PhD for her help in reference searching. We thank Per Aspenberg MD, Philippe Zysset PhD and Dominique Pioletti PhD (who attended the meeting) for their insightful comments. The working group and this paper were fully funded by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), a Belgian not-for-profit organisation. ESCEO was responsible for the selection of participants to the preliminary meeting and for the choice of the authors of the manuscript, covering all expenses related to the organisation of the preliminary meeting and the presentation of the outcomes of the working group at the 2016 World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (in Malaga). ESCEO also covered all expenses pertaining to the preparation, writing and submission of the manuscript. ESCEO received unrestricted educational grants from different non-governmental organisations, not-for-profit organisations and commercial partners; amongst them, AgNovos, which is a company interested in the development and marketing of devices, aimed at reducing the risk of hip fractures in patients with prevalent fractures. None of the commercial partners was involved in the organisation of the ESCEO Working Group, which prepared this manuscript and was not part of the writing or review team of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
SF received consulting fees from Agnovos, Amgen, Eli Lilly, MSD, Radius Health and UCB.
JPD was granted travel supports from Amgen.
JAK reports grants from Amgen, Lilly and Radius Health; non-financial support from Medimaps; non-financial support from Asahi; and institutional support from AgNovos.
JYR received advisory board or speaker fees from Amgen, Analis, Asahi-Kasei, Danone, Ebewee, Eli Lilly, Endocyte, GlaxoSmithKline, IBSA-Genevrier, MSD, Merckle, Negma, Novartis, Novo-Nordisk, NPS, Nycomed-Takeda, PharmEvo, Radius Health, Roche, Rottapharm, Servier, Teijin, Teva, Theramex, UCB, Will Pharma, Wyeth and Zodiac.
MLB has nothing to disclose in relation with the present paper.
JMK received consulting or speaker fees from Amgen, Eli Lilly and Servier.
JMF received consulting or speaker fees from Amgen, Eli Lilly and Servier.
AK received consulting and speaker fees from Agnovos, Amgen, Eli Lilly, Novartis, Novo-Nordisk, Roche, Servier, Teva, Theramex, BioMEt and Dfine.
RR received fees for advisory board or lectures from Danone, Labatec, Nestlé, ObsEva and Radius Health.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s11657-017-0319-2.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ferrari, S., Reginster, JY., Brandi, M.L. et al. Unmet needs and current and future approaches for osteoporotic patients at high risk of hip fracture. Arch Osteoporos 11, 37 (2016). https://doi.org/10.1007/s11657-016-0292-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-016-0292-1