Abstract
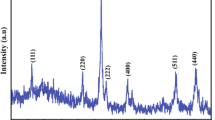
Single-phase manganese oxide, α-Mn2O3 , nanoparticles have been prepared successfully using different amounts of 2w/v% polyvinylpyrrolidone (PVP) via co-precipitation. The samples prepared with 1 ml, 2 ml, 5 ml and 10 ml PVP are represented as S1, S2, S3 and S4, respectively. The effect of PVP amount on the structural, microstructural, magnetic and optical properties was systematically investigated. Rietveld refinement of the x-ray diffraction patterns revealed the single-phase formation of α-Mn2O3 nanoparticles. The average crystallite sizes of the particles was found to be minimum for S2 with lowest lattice parameter and highest strain. High-resolution field emission scanning electron microscopy confirmed the smallest size of S2 with spherical morphology and smooth surfaces. Energy dispersive x-ray spectroscopy and maps showed uniform distribution of the elements favouring the Mn2O3 composition. Raman and Fourier transform infrared spectra displayed characteristic bands corresponding to α-Mn2O3. The magnetic susceptibility revealed the antiferromagnetic nature of α-Mn2O3 nanoparticles with Néel temperature, TN ~ 80.6 K for S2. The increase in PVP amount above 2 ml increased the TN as well as the magnetic frustration. The band gap was found to be maximum (1.8 eV) for S2 nanoparticles. Briefly, the smallest size nanoparticles with spherical shape and smooth surfaces were obtained for 2 ml PVP with the lowest magnetic frustration and highest band gap indicating the optimum amount of PVP to be 2 ml. Thereby, the results have revealed the limiting behaviour of polyvinylpyrrolidone chains operating during synthesis.











Similar content being viewed by others
References
X. Niu, H. Wei, K. Tang, W. Liu, G. Zhao, and Y. Yang, Solvothermal Synthesis of 1D Nanostructured Mn2O3: Effect of Ni2+ and Co2+ Substitution on the Catalytic Activity of Nanowires. RSC Adv. 5, 66271 (2015).
V. Subramanian, H. Zhu, and B. Wei, Nanostructured MnO2: Hydrothermal Synthesis and Electrochemical Properties as a Supercapacitor Electrode Material. J. Power Sources 159, 361 (2006).
R.A.P. Ribeiro, M.C. Oliveira, E. Longo, S.R. de Lazaro, R. Nikam, P.S. Goyal, S. Radha, and S. Rayaprol, Magnetism and DFT Calculations for Understanding Magnetic Ground State of Fe Doped Mn2O3. J. Alloys Compd. 861, 158567 (2021).
P. Amsaveni, A. Nivetha, C. Sakthivel, C.S. Philip, and I. Prabha, Effectiveness of Surfactants for Unique Hierarchical Mn2O3 Nanomaterials as Enhanced Oxidative Catalysts, Antibacterial Agents, and Photocatalysts. J. Phys. Chem. Solids 144, 109429 (2020).
S. Gnanam and V. Rajendran, Facile Hydrothermal Synthesis of Alpha Manganese Sesquioxide (α-Mn2O3) Nanodumb-Bells: Structural, Magnetic, Optical and Photocatalytic Properties. J. Alloys Compd. 550, 463 (2013).
S.K. Ghosh, Diversity in the Family of Manganese Oxides at the Nanoscale : From Fundamentals to Applications. ACS Omega 5, 25493–25504 (2020).
N.E. Rajeevan, P.P. Pradyumnan, R. Kumar, D.K. Shukla, S. Kumar, A.K. Singh, S. Patnaik, S.K. Arora, and I.V. Shvets, Magnetoelectric Properties of BixCo2-XMnO4 (0≤x≤0.3). Appl. Phys. Lett. 92, 111 (2008).
S.K. Sharma, R. Kumar, S. Kumar, V.V. Siva Kumar, M. Knobel, V.R. Reddy, A. Banerjee, and M. Singh, Magnetic Study of Mg0.95Mn0.05Fe2O4 Ferrite Nanoparticles. Solid State Commun. 141, 203 (2007).
G. Anjum, R. Kumar, S. Mollah, D. K. Shukla, S. Kumar, and C. G. Lee, Structural, Dielectric, and Magnetic Properties of La0.8Bi 0.2Fe1-XMnxO3 (0.0≤x≤0.4) Multiferroics, J. Appl. Phys. 107, 0 (2010).
M.S. Anwar, S. Kumar, F. Ahmed, N. Arshi, G.W. Kim, and B.H. Koo, Above Room Temperature Magnetic Transition and Magnetocaloric Effect in La0.66Sr0.34MnO3. J. Korean Phys. Soc. 60, 1587 (2012).
S.A. Ansari, N. Parveen, H.M. Kotb, and A. Alshoaibi, Hydrothermally Derived Three-Dimensional Porous Hollow Double-Walled Mn2O3 Nanocub Es as Superior Electrode Materials for Supercapacitor Applications. Electrochim. Acta 355, 136783 (2020).
L. Suryanti, S.E.I. Suryani, H. Hartatiek, N. Nasikhudin, J. Utomo, A. Taufiq, R. Suryana, Z. Aspanut, and M. Diantoro, The Effect of Mn2O3 Nanoparticles on Its Specific Capacitance of Symmetric Supercapacitors FC-ZnO-x (Mn2O3). Mater. Today Proc. 44, 3355 (2021).
M.S. Anwar, S. Kumar, F. Ahmed, N. Arshi, G.W. Kim, C.G. Lee, and B.H. Koo, Large Magnetic Entropy Change in La0.55Ce0.2Ca0.25MnO3 Perovskite. J. Magn. 16, 457 (2011).
M.S. Anwar, S. Kumar, F. Ahmed, S.N. Heo, G.W. Kim, and B.H. Koo, Study of Magnetic Entropy Change in La0.65Sr0.35Cu0.1Mn0.9O3 Complex Perovskite. J. Electroceramics 30, 46 (2013).
J.H. Mokkath, M. Jahan, M. Tanaka, S. Tominaka, and J. Henzie, Temperature-Dependent Electronic Structure of Bixbyite α-Mn2O3 and the Importance of a Subtle Structural Change on Oxygen Electrocatalysis. Sci. Technol. Adv. Mater. 22, 141 (2021).
A. Indra, P.W. Menezes, F. Schuster, and M. Driess, Significant Role of Mn ( III ) Sites in e 1 g Configuration in Manganese Oxide Catalysts for Efficient Artificial Water Oxidation. J. Photochem. Photobiol. B Biol. 152, 156 (2015).
S. Geller, Structures of Alpha-Mn2O3, (Mn0.983Fe0.017)2O3 and (Mn0.37Fe0.63)2O3 and Relation to Magnetic Ordering. Acta Cryst. B 27, 821 (1971).
M. Sharrouf, R. Awad, M. Roumié, and S. Marhaba, Structural, Optical and Room Temperature Magnetic Study of Mn2O3 Nanoparticles. Mater. Sci. Appl. 6, 850 (2015).
M. Regulski, R. Przeniosło, I. Sosnowska, D. Hohlwein, and R. Schneider, Neutron Diffraction Study of the Magnetic Structure of α-Mn2O3. J. Alloys Compd. 362, 236 (2004).
M. Karuppaiah, P. Sakthivel, S. Asaithambi, R. Murugan, G. Anandha, R. Yuvakkumar, and G. Ravi, Solvent Dependent Morphological Modification of Micro-Nano Assembled Mn2O3/NiO Composites for High Performance Supercapacitor Applications. Ceram. Int. 45, 4298 (2019).
H. Ju, X.D. Liu, C.Y. Tao, F. Yang, X.L. Liu, X. Luo, and L. Zhang, Prussian Blue Analogue Derived Low-Crystalline Mn2O3/Co3O4 as High- Performance Supercapacitor Electrode. J. Alloys Compd. 856, 157134 (2021).
B.L. Vijayan, S.G. Krishnan, N.K.M. Zain, M. Harilal, A. Yar, I.I. Misnon, J.O. Dennis, M.M. Yusoff, and R. Jose, Large Scale Synthesis of Binary Composite Nanowires in the Mn2O3-SnO2 System with Improved Charge Storage Capabilities. Chem. Eng. J. 327, 962 (2017).
Z.H. Wang, D.Y. Geng, W.J. Hu, W.J. Ren, and Z.D. Zhang, Magnetic Properties and Exchange Bias in Mn2O3∕Mn3O4 Nanoclusters. J. Appl. Phys. 105, 07A315 (2009).
E. Cockayne, I. Levin, H. Wu, and A. Llobet, The Magnetic Structure of Bixbyite α-Mn2O3: A Combined Density Functional Theory DFT+U and Neutron Diffraction Study. Phys. Rev. B 87, 184413 (2013).
S. Jana, Advances in Nanoscale Alloys and Intermetallics: Low Temperature Solution Chemistry Synthesis and Application in Catalysis. Dalt. Trans. 44, 18692 (2015).
M.F. Silva, L.A.S. De Oliveira, M.A. Ciciliati, M.K. Lima, F.F. Ivashita, D.M. Fernandes De Oliveira, A.A.W. Hechenleitner, and E.A.G. Pineda, The Effects and Role of Polyvinylpyrrolidone on the Size and Phase Composition of Iron Oxide Nanoparticles Prepared by a Modified Sol-Gel Method. J. Nanomater. 2017, 17 (2017).
P. Díaz-Núñez, J. González-Izquierdo, G. González-Rubio, A. Guerrero-Martínez, A. Rivera, J.M. Perlado, L. Bañares, and O. Peña-Rodríguez, Effect of Organic Stabilizers on Silver Nanoparticles Fabricated by Femtosecond Pulsed Laser Ablation. Appl. Sci. 7, 793 (2017).
N. Arshi, F. Ahmed, S. Kumar, M.S. Anwar, B.H. Koo, and C.G. Lee, Comparative Study of the Ag/PVP Nanocomposites Synthesized in Water and in Ethylene Glycol. Curr. Appl. Phys. 11, S346 (2011).
J. Tientong, S. Garcia, C.R. Thurber, and T.D. Golden, Synthesis of Nickel and Nickel Hydroxide Nanopowders by Simplified Chemical Reduction. J. Nanotechnol. 2014, 1 (2014).
A. Khort, S. Roslyakov, and P. Loginov, Solution Combustion Synthesis of Single-Phase Bimetallic Nanomaterials. Nano-Struct. Nano-Objects 26, 100727 (2021).
J. Li, K. Inukai, Y. Takahashi, A. Tsuruta, and W. Shin, Effect of PVP on the Synthesis of High-Dispersion Core-Shell Barium-Titanate–Polyvinylpyrrolidone Nanoparticles. J. Asian Ceram. Soc. 5, 216 (2017).
J. Rodríguez-carvajal, Recent Developments of the Program FULLPROF, in Commission on Powder Diffraction, (IUCr). Newsl. 26, 12 (2001).
G. Will, Powder Diffraction the Rietveld Method and the Two Stage Method to Determine and Refine Crystal Structures from Powder Diffraction Data (Berlin Heidelberg: Springer-Verlag, 2006).
Y. Li, L. Liu, L. Liu, Y. Liu, H. Zhang, and X. Han, Efficient Oxidation of Phenol by Persulfate Using Manganite as a Catalyst. J. Mol. Catal. A Chem. 411, 264 (2016).
Y. Son, P.T.M. Bui, H. Lee, M.S. Akhtar, D.K. Shah, and O. Yang, A Rapid Synthesis of Mesoporous Mn2O3 Nanoparticles for Supercapacitor Applications. Coatings 9, 631 (2019).
B.H. Toby, Chapter 4.7. Rietveld Refinement, in International Tables for Crystallography Volume H: Powder Diffraction (Wiley, 2019), pp. 465–472.
B.H. Toby, R Factors in Rietveld Analysis: How Good is Good Enough? Powder Diffr. 21, 67 (2006).
L.B. Mccusker, R.B. Von Dreele, D.E. Cox, D. Louer, and P. Scardi, Rietveld Refinement Guidelines. J. Appl. Cryst. 32, 36 (1999).
K. Kumari, R.N. Aljawfi, Y.S. Katharria, S. Dwivedi, K.H. Chae, R. Kumar, A. Alshoaibi, P.A. Alvi, S. Dalela, and S. Kumar, Study the Contribution of Surface Defects on the Structural, Electronic Structural, Magnetic, and Photocatalyst Properties of Fe: CeO2 Nanoparticles. J. Electron Spectros. Relat. Phenomena 235, 29 (2019).
F. Jiao, A. Harrison, A.H. Hill, and P.G. Bruce, Mesoporous Mn2O3 and Mn3O4 with Crystalline Walls. Adv. Mater. 19, 4063 (2007).
R. Najjar, R. Awad, and A.M. Abdel-Gaber, Physical Properties of Mn2O3 Nanoparticles Synthesized by Co-Precipitation Method at Different PH Values. J. Supercond. Nov. Magn. 32, 885 (2019).
S. Kumar, K. Kumari, F.A. Alharthi, F. Ahmed, R. Naji, P.A. Alvi, R. Kumar, M. Hashim, and S. Dalela, Investigations of TM (Ni, Co) Doping on Structural, Optical and Magnetic Properties of CeO2 Nanoparticles. Vacuum 181, 109717 (2020).
D. Nath and R. Das, Experimental (XRD) and Theoretical (DFT) Analysis for Understanding the Influence of SHI Irradiation on the Stacking Fault Energy in CdSe Nanocrystals. J. Alloys Compd. 879, 160456 (2021).
A.J. Deotale and R.V. Nandedkar, Correlation Between Particle Size, Strain and Band Gap of Iron Oxide Nanoparticles. Mater. Today Proc. 3, 2069 (2016).
I.M. Alibe, K.A. Matori, H.A.A. Sidek, Y. Yaakob, U. Rashid, A. Mustapha, M. Hafiz, M. Zaid, S. Nasir, and M. Mohammed, Effects of Polyvinylpyrrolidone on Structural and Optical Properties of Willemite Semiconductor Nanoparticles by Polymer Thermal Treatment Method. J. Therm. Anal. Calorim. 136, 2249 (2019).
S. Dagar, A. Hooda, S. Khasa, and M. Malik, Rietveld Refinement, Dielectric and Magnetic Properties of NBT-Spinel Ferrite Composites. J. Alloys Compd. 806, 737 (2019).
S. Bernardini, F. Bellatreccia, A.C. Municchia, G. Della Ventura, and A. Sodo, Raman Spectra of Natural Manganese Oxides. J Raman Spectrosc. 50, 1 (2019).
A.C. Ferrari, S.E. Rodil, and J. Robertson, Interpretation of Infrared and Raman Spectra of Amorphous Carbon Nitrides. Phys. Rev. B 67, 155306 (2003).
C.M. Julien, M. Massot, and C. Poinsignon, Lattice Vibrations of Manganese Oxides Part I. Periodic Structures. Spectrochim. Acta Part A 60, 689 (2004).
A.H.A.M. Videla, L. Osmieri, R.A.M. Esfahani, J. Zeng, C. Francia, and S. Specchia, The Use of C-MnO2 as Hybrid Precursor Support for a Pt/C-MnxO1+x Catalyst with Enhanced Activity for the Methanol Oxidation Reaction (MOR). Catalysts 5, 1399 (2015).
F. Buciuman, F. Patcas, R. Craciun, and D.R.T. Zahn, Vibrational Spectroscopy of Bulk and Supported Manganese Oxides. Phys. Chem. Chem. Phys. 1, 185 (1999).
L. Slavov, M.V. Abrashev, T. Merodiiska, C. Gelev, R.E. Vandenberghe, I. Markova-Deneva, and I. Nedkov, Raman Spectroscopy Investigation of Magnetite Nanoparticles in Ferrofluids. J. Magn. Magn. Mater. 322, 1904 (2010).
L. Jayaselvan and C. Gnanasambandam, Structural, Optical and Phase Formation Modifications by Varying Precursor Temperature on Mn2O3 Nanoparticles Prepared by Microwave Assisted Precipitation. AIP Conf. Proc. 2311, 080008 (2020).
A. Filtschew, K. Hofmann, and C. Hess, Ceria and Its Defect Structure : New Insights from a Combined Spectroscopic Approach. J. Phys. Chem. C 120, 6694–6703 (2016).
A. Vijayamari, K. Sadayandi, S. Sagadevan, and P. Singh, A Study of Optical, Surface Morphological and Electrical Properties of Manganese Oxide Nanoparticles. J. Mater. Sci. Mater. Electron. 28, 2739 (2017).
S. Li, Y.H. Lin, B.P. Zhang, Y. Wang, and C.W. Nan, Controlled Fabrication of BiFeO3 Uniform Microcrystals and Their Magnetic and Photocatalytic Behaviors. J. Phys. Chem. C 114, 2903 (2010).
S. Kumar, M. Sharma, R.R.N., K.H. Chae, R. Kumar, S. Dalela, A. Alshoaibi, F. Ahmed, and P.A. Alvi, Tailoring the Structural, Electronic Structure and Optical Properties of Fe: SnO2 Nanoparticles, J. Electron Spectros. Relat. Phenomena 240, 146934 (2020).
M. Singh, M. Goyal, and K. Devlal, Size and Shape Effects on the Band Gap of Semiconductor Compound Nanomaterials. J. Taibah Univ. Sci. 12, 1 (2018).
Acknowledgments
Support for this project comes from the National Research Foundation of Korea grant funded by the Korean government (No. 2018R1D1A1B07046937).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumari, K., Kumar, S., Huh, SH. et al. Effect of PVP Assisted Growth of α-Mn2O3 Nanoparticles on the Structural, Microstructural, Magnetic and Optical Properties. J. Electron. Mater. 51, 5842–5856 (2022). https://doi.org/10.1007/s11664-022-09804-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-022-09804-3