Abstract
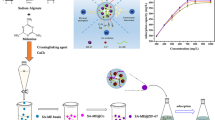
The demand for lithium has been steadily growing in recent years due to the boom of electric cars. High purity lithium is commonly used in the manufacture of battery grade lithium electrolyte. Sulfate residuals originating from acid leaching of lithium ores must be limited to below 20 mg·L−1 during refining. There are methods to remove sulfate such as membrane processing and chemical precipitation using barium salts. However, membrane separation is unable to achieve the required purity while chemical precipitation often causes secondary contamination with barium and requires extra filtration processes that lead to increased processing costs. In this study, we developed a polymeric matrix entrapped with barium ions as a novel adsorbent to selectively adsorb sulfate in aqueous solutions. The adsorbent was prepared by dropwise injection method where alginate droplets were crosslinked with barium to form hydrogel microcapsules. In a typical scenario, the microcapsules had a diameter of 3 mm and contained 5 wt-% alginate. The microcapsules could successfully reduce sulfate concentration in a solution from 100 to 16 mg·L−1, exceeding the removal target. However, the microcapsules were mechanically unstable in the presence of an excess amount of sulfate. Hence, calcium ions were added as a secondary cross-linking agent to improve the integrity of the microcapsules. The two-step Ca/Ba@alginate microcapsules showed an exceptional adsorption performance, reducing the sulfate concentration to as low as 0.02 mg·L−1. Since the sulfate selective microcapsules can be easily removed from the aqueous system and do not result in secondary barium contamination, these Ca/Ba@alginate adsorbents will find applications in ultra-refining of lithium in industry.

Similar content being viewed by others
References
Hykawy J, Chudnovsky T. Industry report//Lithium. Stormcrow technical report. 2015
Grosjean C, Miranda P H, Perrin M, Poggi P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renewable & Sustainable Energy Reviews, 2012, 16(3): 1735–1744
Kesler S E, Gruber P W, Medina P A, Keoleian G A, Everson M P, Wallington T J. Global lithium resources: relative importance of pegmatite, brine and other deposits. Ore Geology Reviews, 2012, 48: 55–69
Chen Y, Tian Q, Chen B, Shi X, Liao T. Preparation of lithium carbonate from spodumene by a sodium carbonate autoclave process. Hydrometallurgy, 2011, 109(2): 43–46
Peiró L T, Méndez G V, Ayres R U. Lithium: sources, production, uses, and recovery outlook. JOM, 2013, 65(8): 986–996
Buckley D, Genders J D. Method of making high purity lithium hydroxide and hydrochloric acid. US Patent, 20110044882A1, 2011-02-24
Livent. Lithium hydroxide monohydrate, battery grade. Datasheet. 2018
Wang L K, Vaccari D A, Li Y, Shammas N K. Physicochemical Treatment Processes. In: Handbook of Environmental Engineering. New Delhi: Humana Press, 2005, 3: 141–197
Sarkar S, Sengupta A K, Prakash P. The Donnan membrane principle: opportunities for sustainable engineered processes and materials. Environmental Science & Technology, 2010, 44(4): 1161–1166
Tang C Y, Yang Z. Transmission electron microscopy (TEM). Membrane Characterization, 2017, 145–159
Lin S. Water and wastewater calculations manual 3rd edition, 2014
Chen G G, Luo G S, Xu J H, Wang J D. Membrane dispersion precipitation method to prepare nanoparticles—A Review. Powder Technology, 2004, 139(2): 180–185
Wong D C Y, Jaworski Z, Nienow A W. Effect of ion excess on particle size and morphology during barium sulfate precipitation: an experimental study. Chemical Engineering Science, 2001, 56(3): 727–734
Lee K Y, Mooney D J. Alginate: properties and biomedical applications. Progress in Polymer Science, 2012, 37(1): 106–126
Lu D R, Xiao C M, Xu S J. Starch-based completely biodegradable polymer materials. Express Polymer Letters, 2009, 3(6): 366–375
Li Z, Ramay H R, Hach K D, Xiao D, Zhang M. Chitosan-alginate hybrid scaffolds for bone tissue engineering. Biomaterials, 2005, 26(18): 3919–3928
Fiol N, Escudero C, Poch J, Villaescus I. Preliminary studies on Cr (VI) removal from aqueous solution using grape stalk wastes encapsulated in calcium alginate beads in a packed bed up-flow column. Reactive & Functional Polymers, 2006, 66(8): 795–807
Lee B B, Ravindra P, Chan E S. Size and shape ofcalcium alginate beads produced by extrusion dripping. Chemical Engineering & Technology, 2013, 36(10): 1627–1642
Chan E S, Lee B B, Ravindra P, Poncelet D. Prediction models for shape and size of ca-alginate macrobeads produced through extrusion-dripping method. Journal of Colloid and Interface Science, 2009, 338(1): 63–72
Papageorgiou S K, Kouvelos E P, Katsaros F K. Calcium alginate beads from Laminaria digitata for the removal of Cu2+ and Cd2+ from dilute aqueous metal solutions. Desalination, 2008, 224(1–3): 293–306
Larsen B E, Bjørnstad J, Pettersen E O, Tønnesen H H, Melvik J E. Rheological characterization of an injectable alginate gel system. BMC Biotechnology, 2015, 15(1): 29
Fogler H S. Elements of Chemical Reaction Engineering. 5th ed. New Jersey: Prentice Hall, 2016, chapter 14: 1–10
Cai W, Chen R, Yang Y, Yi M, Xiang L. Removal of SO42− from Li2CO3 by recrystallization in Na2CO3 solution. Crystals, 2018, 8(1): 19–26
Mørch Ý A, Donati I, Strand B L, Skjåk-Bræk G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules, 2006, 7(5): 1471–1480
Fathy M, Moghny T A, Awadallah A E, Au El-Bellihi A. Study the adsorption of sulfates by high cross-linked polystyrene divinylbenzene anion-exchange resin. Applied Water Science, 2017, 7(1): 309–313
Largitte L, Pasquier R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chemical Engineering Research & Design, 2016, 109: 495–504
Acknowledgements
This work was supported by the Department of Chemical Engineering at The University of Melbourne.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, L., Chen, K., Chen, G.Q. et al. Development of barium@alginate adsorbents for sulfate removal in lithium refining. Front. Chem. Sci. Eng. 15, 198–207 (2021). https://doi.org/10.1007/s11705-020-1968-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-020-1968-z