Abstract
In a search for biologically active phenolics, a hydroalcoholic extract from the hairy roots of Lactuca virosa was fractionated by chromatographical methods. The procedure led to the isolation of a substantial amount of 3,5-dicaffeoylquinic acid (3,5-DCQA)—a potent free radical scavenger. An analytical RP-HPLC separation of the hydroalcoholic extract from the hairy roots allowed identification of further hydroxycinnamates: caftaric acid (CTA), chlorogenic acid (5-CQA) and cichoric acid (DCTA), as well as small amounts of unbound phenolic acids. A time course of growth and caffeic acid derivatives accumulation in the hairy root culture was also investigated. The highest contents of the compounds in the examined roots were detected at the logarithmic phase of growth. The average content of 3,5-DCQA in the roots (ca. 2.5% DW) was at least one order of magnitude higher than that found in roots of Lactuca species and callus culture of L. virosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydroxycinnamates are a group of phenolic compounds comprising free acids—hydroxyderivatives of cinnamic acid (e.g.: caffeic, ferulic, synapic and p-coumaric acids) and their conjugates. One of the commonest conjugates is 5-CQA also known as chlorogenic acid. Dicaffeoylquinic acids (diCQAs) along with tri- and tetra-caffeoylquinic derivatives as well as mono- and di-caffeoyl conjugates with some dicarboxylic aliphatic acids (e.g.: tartaric or succinic acid) are characteristic of Asteraceae plants. The compounds are potent radical scavengers and lipid peroxidation inhibitors (Ohnishi et al. 1994; Kim and Lee 2005; Olmos et al. 2008) and also play a role as infection and insect infestation inhibiting factors in plants (Kodoma et al. 1998; Leiss et al. 2009). Lettuce (Lactuca sativa L.) plants are a rich dietary source of hydroxycinnamates (Degl’Innocenti et al. 2008; Llorach et al. 2008). Lactuca virosa L. (Asteraceae, Lactuceae)—poisonous (bitter) lettuce, which due to its multiple virus resistance has been used in breeding programs to introduce the resistance genes into commercial varieties of the garden lettuce (Tamaki et al. 1995), has not been studied before with respect to hydroxycinnamate content. The whole L. virosa plant as well as its leaves and latex (known as lettuce opium) reportedly possess medicinal properties and have been mentioned in different Pharmacopoeias (Stojakowska et al. 1999) as antitussic and sedative remedies. Analgesic and sedative activities of lactucin-like guaianolides, sesquiterpene lactones characteristic of L. virosa plants, have been recently confirmed by Wesołowska et al. (2006). The lactucin-like guaianolides were absent from undifferentiated tissue cultures of the plant (Stojakowska et al. 1994). Major secondary metabolites accumulated by the callus and suspension cultures were neolignan glycosides (Stojakowska and Kisiel 2000). From a hairy root culture of L. virosa sesquiterpene lactones characteristic of roots of the intact plant, sterols and pentacyclic triterpenols were isolated (Kisiel et al. 1995). The presence of terpenoids, however, did not explain an activity of a hydroalcoholic extract from the hairy roots in scavenging of DPPH free radical (data not shown). This observation prompted us to reinvestigate the culture in a search of antiradical agents.
Materials and methods
Plant material
Seeds of botanically verified L. virosa plants, collected from natural population were delivered by Botanical Garden in Nantes (France). The seeds were surface sterilized and aseptically germinated on half-strength MS medium (Murashige and Skoog 1962), solidified with 0.8% agar.
Roots and aerial parts from plants of the genus Lactuca were collected in the first year of their vegetation periods in the Garden of Medicinal Plants, Institute of Pharmacology, Polish Academy of Sciences, Kraków, Poland.
Callus induction and culture
Leaf explants of L. virosa aseptic seedlings (ca. 1 cm long) were inoculated onto a solidified MS medium containing 2,4-D (4.52 μM l−1), kinetin (1.39 μM l−1) and 3% sucrose (pH adjusted to 5.8, before autoclaving) and maintained at 28°C, under continuous illumination (40 μmol m−2 s−1, cool white fluorescent tubes) to induce calli. The calli were then transferred to a fresh MS agar medium of the same composition as the induction medium, for further growth. Temperature and illumination conditions of the culture remained unaltered. The tissue was subcultured every 6 weeks by inoculating ca. 2.0 g fresh weight of the callus onto the fresh nutrient medium. For phytochemical investigation the calli were collected at the end of 6 week culture period and dried at room temperature.
Hairy roots
A hairy root culture of L. virosa, transformed by Agrobacterium rhizogenes, was established from aseptic seedlings of the plant obtained from the seeds of known wild origin, delivered by the Botanical Garden in Nantes (France). A. rhizogenes strain LBA 9402, containing agropine type Ri plasmid pRi 1855, was used in the experiment. Hairy roots were induced on leaf explants excised from the seedlings, and their transformed nature was proved by opine assay and rol B gene detection in plant genomic DNA, as described elsewhere (Stojakowska and Malarz 2000), except for that the REDTag ReadyMix PCR reaction mix (Sigma-Aldrich Co., St. Louis, MO, USA) replaced the corresponding components of the amplification reaction mixture. The transformed roots were cultivated in a modified liquid MS medium, containing ½ strength macronutrients and 3% sucrose, on a gyrotory shaker (110 r.p.m.), at 25°C, in the dark. The nutrient medium was initially supplemented with 500 mg l−1 of cefotaxime to obtain bacteria-free culture. The roots were subcultured every 4 weeks by inoculating 0.5 g of a fresh biomass in 250 ml Erlenmeyer flask containing 30 ml of the fresh medium. A time course experiment was performed by harvesting roots every 5 days during 35 days of culture. The experiment was done in triplicate. A DW of roots, as well as hydroxycinnamate contents were estimated.
Chemicals and solvents
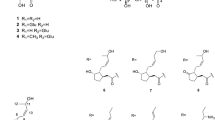
CTA (1, purity >97% was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). DCTA (2, purity >98% by HPLC), 5-CQA (3, purity >97% by HPLC) and cynarin (1, 3-dicaffeoylquinic acid, purity >99% by HPLC) were purchased from Roth (Karlsruhe, Germany). A standard sample of 3,5-DCQA (4) was isolated in our laboratory from L. virosa root culture, and identified by comparison of its spectral (1H NMR, 300.08 MHz) and physical ([α] D ) data with those found in the literature (Basnet et al. 1996; Pauli et al. 1998; Islam et al. 2002). The compound 4 was of purity 90.0% (by HPLC). MeOH, n-hexane, chloroform and n-butanol of analytical grade were purchased from POCh S.A. (Gliwice, Poland). Water was purified by a Mili-Q system (Milipore Corp., Bedford, MA, USA). MeOH and MeCN of HPLC grade as well as formic acid and glacial acetic acid were purchased from Merck (Darmstadt, Germany).
Isolation of 3,5-DCQA from hairy roots
Dried and pulverized roots (26 g) were exhaustively extracted with 80% MeOH at room temperature. The extract was concentrated in vacuo providing an oily residue (10.5 g) which was suspended in water and successively extracted with n-hexane, chloroform and n-butanol. The butanolic fraction (2.2 g) was subjected to CC on Polyamide with MeOH as an eluent and subsequently to CC on Sephadex LH-20 (Pharmacia Biotech, Uppsala, Sweden) eluted with a MeOH/H2O mixture (4:1, v/v). This procedure led to the isolation of a substantial amount (217 mg) of 3,5-DCQA. Its optical rotation was determined with a PolAAr31 automatic polarimeter (Optical Activity LTD, England) and 1H NMR spectrum was measured in deuterated MeOH on a Varian Mercury-VX 300 spectrophotometer.
Analytical RP-HPLC separation and quantification of hydroxycinnamates
Lyophilised roots (0.05 g) were extracted twice with 12.5 ml of 70% MeOH at room temperature. The combined extracts were evaporated in vacuo and the residue was dissolved in 1 ml of MeOH, left to stand overnight at 4°C, centrifuged (1,1340×g, 5 min) and analysed by RP-HPLC. Analytical RP-HPLC separations of the samples were performed using an Agilent 1200 Series HPLC system (Agilent Technologies, USA) equipped with a Rheodyne manual sample injector, quaternary pump, degasser, column oven and a diode array detector. Chromatographic separations of hydroxycinnamates were carried out at 25°C, on a Zorbax Eclipse XDB-C18 column, 4.6 × 150 mm (Agilent Technologies, USA), with a mobile phase consisting of H2O/HCOOH/CH3COOH 99/0.9/0.1 (solvent A) and MeCN/MeOH/HCOOH/CH3COOH 89/10/0.9/0.1 (solvent B), at a flow rate of 1 ml min−1, using 5 μl injections. The gradient elution conditions described by Spitaler et al. (2006) were applied. The analysis allowed identification of the following hydroxycinnamates of known biological activities present in the plant material [CTA, Rt (6.6 min); 5-CQA, Rt (7.3 min); DCTA, Rt (20.3 min) and 3,5-DCQA, Rt (28.2 min)], by comparison of their retention times and UV spectra with those of authentic samples and co-chromatography with standards. Compounds 1–4 (Fig. 1) were quantified using four point calibration curves, based on peak areas measured at 325 nm, prepared for cynarin, CTA, DCTA and 5-CQA (concentration range 0.02–1.50 mg ml−1).
Quantification of lactucin-like guaianolides
Lyophilised roots (0.1 g) were extracted twice with 10 ml of MeOH at room temperature. The combined extracts were evaporated in vacuo and the residue was dissolved in 1 ml of MeOH, left to stand overnight at 4°C, centrifuged (11,340×g, 5 min) and analysed by RP-HPLC, at 40°C, using the aforementioned equipment. A fractionated sample (5 μl) was injected into a Purospher RP-18e (3 × 125 mm, particle size 5 μm) column (Merck, Darmstadt, Germany) eluted with a mobile phase consisting of water and MeCN, at a flow rate of 1 ml min−1. The gradient elution conditions described by Grass et al. (2006) were applied. The typical retention times of the analysed compounds were as follows: 8-deoxylactucin glycoside (9.0 min), crepidiaside B (10.2 min), 8-deoxylactucin (13.5 min), jacquinelin (15.1 min) and lactucopicrin (26.8 min). Quantification was done by measurement of peak area at 260 nm with reference to the standard curve derived from five concentrations (0.125 to 2.000 mg ml−1) of the guaianolide jacquineline acetate.
Quantification of unbound phenolic acids
Protocatechuic, p-hydroxybenzoic, caffeic, p-coumaric, ferulic and cinnamic acids were quantified, as described elsewhere (Ellnain-Wojtaszek and Zgórka 1999; Ekiert et al. 2009), after extraction of dried biomass of in vitro grown hairy roots with boiling methanol and subsequent RP-HPLC analysis of the obtained extract.
Statistics
All experiments were carried out in duplicate and were repeated at least once. Results are presented as mean values ± standard deviation (Microsoft Excel 2000).
Results and discussions
After 30 days of culture, the biomass of L. virosa hairy roots reached 0.57 g per flask (19 g l−1) and 8 g per flask (267 g l−1) measured as DW and FW, respectively (Fig. 2). A growth index (GI) of roots, calculated as a ratio of a final weight of roots at a harvest time to a weight of inoculum, was 15.8 (on a DW basis). The GI of roots was about two times higher than that of calculated for a suspension culture of L. virosa (Stojakowska and Kisiel 2000) and a transformed root clone described earlier (Stojakowska et al. 1999). After ca. 5 weeks of culture, a growth arrest could be observed in the roots accompanied by a change in tissue colour from bright yellow to grey. The main phenolic secondary metabolite isolated from the culture was 3,5-DCQA—a potent free radical scavenger (Kim and Lee 2005; Park et al. 2009), an inhibitor of lipid peroxidation and haemolysis (Ohnishi et al. 1994) which exerted protective effect against peroxynitrite and other reactive nitrogen species (Olmos et al. 2008) and protected human cells in vitro against LPS or H2O2 induced apoptosis by scavenging intracellular ROS and suppression of caspase-3 activation (Kim et al. 2005; Zha et al. 2007). The compound reportedly possesses inhibitory activities against HIV-1 replication and HIV integrase (Robinson et al. 1996; McDougall et al. 1998). 3,5-DCQA constituted nearly 3% of the DW of the examined roots. A qualitative and quantitative analysis of hydroxycinnamates present in L. virosa hairy roots also showed a presence of 5-CQA (up to 1.5% DW), DCTA (up to 0.7% DW) and a low amount of CTA (up to 0.027% DW) (Fig. 4). According to Nicolle et al. (2004), 5-CQA and DCTA constituted up to 0.61% DW and 1.12% DW of L. sativa aerial parts, respectively. Contribution of DCTA alone to the total antioxidant power (measured as DPPH scavenging activity) of hydroalcoholic extracts from different cultivars of lettuce reached nearly 70%. DiCQAs were also detected as active compounds in Bidens pilosa and Dipsacus asper extracts which exhibited significant scavenging free radical activity (Chiang et al. 2004; Hung et al. 2006). Thus, hydroxycinnamates seem to be responsible for high activity of extracts from L.virosa hairy roots in DPPH scavenging. Although, the contents of hydroxycinnamates in the roots remained relatively stable throughout the culture period (Fig. 3), their maxima could be detected at the day 10th of the culture (at the logarithmic phase of growth). The hydroxycinnamate contents were higher than those found in roots and aerial parts of L. sativa and L. virosa plants grown in the garden (Table 1). A callus culture of L. virosa produced a similar set of hydroxycinnamates but the estimated contents of the compounds were significantly lower (Table 1). In our previous study (Kisiel et al. 1995), we described an isolation of sesquiterpene lactones from hairy roots of L. virosa, however, the data concerning the culture growth and time course of lactucin-like guaianolide accumulation in the roots were not published previously (Fig. 2; Table 2). In the cultured roots, unlike in roots of the intact plant, the sesquiterpene lactones are accumulated mainly as their glucosides. In the beginning of the growth cycle, the ratio of guaianolides which possess highly reactive egzomethylene group, responsible for their numerous biological activities (8-deoxylactucine and its glucoside) to their dihydroderivatives of low activity (jacquinelin and crepidiaside B) was ca. 5:1. At the stationary phase of the culture, the ratio dropped to 2:1. The high ratio in the beginning of the culture growth might be related to the stress-induced defence response of the tissue caused by the damage done during the transfer of inoculum to the fresh medium. The analysed sesquiterpene lactones reached their maximum contents in roots at the different phases of the culture growth and except for that of 8-deoxylactucin glucoside the maxima did not coincide with maximum accumulation of hydroxycinnamates. Low amounts of unbound phenolic acids were also detected in the roots harvested after four-week culture (Table 3).
HPLC separation of hydroxycinnamates from hairy roots of L. virosa (λ = 325 nm): 1 CTA, 2 DCTA, 3 5-CQA, 4 3,5-DCQA. a Separation of a crude extract from the cultured roots; b cochromatography of the crude extract from the roots with CTA standard sample; c cochromatography of the crude extract from the roots with DCTA standard sample
The best known in vitro culture systems for production of hydroxycinnamates are root cultures of Echinacea purpurea (L.) Moench. (Liu et al. 2006; Abbasi et al. 2007; Wu et al. 2007; Jeong et al. 2009). According to Pellati et al. (2004), total phenolics content in roots of E. purpurea plants was 2.3% DW. Adventitious root cultures of E. purpurea investigated by Jeong et al. (2009) accumulated 0.49% DW of CTA, 0.60% DW of 5-CQA and 2.81% DW of DCTA in the optimized culture conditions (GI 10.9). Hairy roots of the plant produced 12.9 g l−1 DW of biomass after 35-day culture. Yields of hydroxycinnamates were similar to those from adventitious roots (Abbasi et al. 2007). Elicited cell cultures of E. purpurea produced wide array of phenolic compounds (lignans, neolignans, acetophenone derivatives and phenolic glycosides) partly accumulated in cells and partly liberated to the culture medium. Caffeoyltartaric and caffeoylquinic acids, however, were not found in the cultures (Li and Barz 2006). Although E. purpurea root cultures were better CTA and DCTA producers than L. virosa hairy roots, their capability to accumulate caffeoylquinic derivatives was limited. Hairy roots of L. virosa accumulated nearly three times more of 5-CQA than root cultures of E. purpurea and up to 3% DW of 3,5-DCQA which was absent from E. purpurea roots. Undifferentiated cultures of the both plant species were poor sources of hydroxycinnamates. Aerial parts of Lactuca sativa L. intact plants accumulated DCTA as major caffeic acid derivative and only minor amounts of 3,5-DCQA (Degl’Innocenti et al. 2008; Ribas-Agustí et al. 2011). In the roots of L. sativa and L. virosa plants cultivated in our garden, contents of DCTA were similar to those of 3,5-DCQA (Table 1). Moreover, the DCTA contents in roots were much lower than those found in the aerial parts of the plants. In a callus culture of L. sativa var. crispa L. (Tamura et al. 2006) 3,5-DCQA predominated (leaves of the parent plant contained 3.8 times less of 3,5-DCQA than the cultured cells). Similar change in hydroxycinnamate accumulation pattern (domination of 3,5-DCQA) was observed in our callus and root cultures (Table 1).
Hairy root cultures of L. virosa are efficient producers of caffeic acid derivatives of high antioxidant and chemopreventive activity. Their unique features are capability to synthesize 3,5-DCQA as a sole diCQA which makes an isolation of the pure compound easy, and simultaneous accumulation of other chemopreventive hydroxycinnamates as well as anti-inflammatory active (Cavin et al. 2005) sesquiterpene lactones.
Abbreviations
- DW:
-
Dry weight
- FW:
-
Fresh weight
- 5-CQA:
-
Chlorogenic acid
- 3,5-DCQA:
-
3,5-Dicaffeoylquinic acid
- CTA:
-
Caftaric acid
- DCTA:
-
Cichoric acid
- DPPH:
-
2, 2-Diphenyl-1-picrylhydrazyl
- 2, 4-D:
-
2, 4-Dichlorophenoxyacetic acid
- RP-HPLC:
-
Reversed-phase high-performance liquid chromatography
- CC:
-
Conventional column chromatography
- MeOH:
-
Methanol
- MeCN:
-
Acetonitrile
References
Abbasi BH, Tian C-L, Murch SJ, Saxena PK, Liu C-Z (2007) Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep 26:1367–1372
Basnet P, Matsushige K, Hase K, Kadota S, Namba T (1996) Four di-O-caffeoyl quinic acid derivatives from propolis. Potent hepatoprotective activity in experimental liver injury models. Biol Pharm Bull 19:1479–1484
Cavin C, Delannoy M, Malnoe A, Debefve E, Touche A, Courtois D, Schilter B (2005) Inhibition of the expression and activity of cyclooxygenase-2 by chicory extract. Biochem Biophys Res Commun 327:742–749
Chiang YM, Chuang D-Y, Wang S-Y, Kuo Y-H, Tsai P-W, Shyur L-F (2004) Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J Ethnopharm 95:409–419
Degl’Innocenti E, Pardossi A, Tattini M, Guidi L (2008) Phenolic compounds and antioxidant power in minimally processed salad. J Food Biochem 32:642–653
Ekiert H, Szewczyk A, Kuś A (2009) Free phenolic acids in Ruta graveolens L. in vitro culture. Pharmazie 64:694–696
Ellnain-Wojtaszek M, Zgórka G (1999) High performance liquid chromatography and thin-layer chromatography of phenolic acids from Ginkgo biloba L. leaves collected within vegetative period. J Liq Chrom Tech 22:1457–1471
Grass S, Zidorn C, Blattner FR, Stuppner H (2006) Comparative molecular and phytochemical investigation of Leontodon autumnalis (Asteraceae, Lactuceae) populations from Central Europe. Phytochemistry 67:122–131
Hung TM, Na MK, Thuong PT, Su ND, Sok DE, Song KS, Seong YH, Bae KH (2006) Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper Wall. J Ethnopharm 108:188–192
Islam MS, Yoshimoto M, Yahara S, Okuno S, Ishiguro K, Yamakawa O (2002) Identification and characterization of foliar polyphenolic composition in sweetpotato (Ipomea batatas L.) genotypes. J Agric Food Chem 50:3718–3722
Jeong J-A, Wu C-H, Murthy HN, Hahn E-J, Paek K-Y (2009) Application of an airlift bioreactor system for the production of adventitious root biomass and caffeic acid derivatives of Echinacea purpurea. Biotechnol Bioprocess Eng 14:91–98
Kim HJ, Lee YS (2005) Identification of new dicaffeoylquinic acids from Chrysanthemum morifolium and their antioxidant activities. Planta Med 71:871–876
Kim S–S, Park R-Y, Jeon H-J, Kwon Y-S, Chun W (2005) Neuroprotective effects of 3,5-dicaffeoylquinic acid on hydrogen peroxide-induced cell death in SH-SY5Y cells. Phytother Res 19:243–245
Kisiel W, Stojakowska A, Malarz J, Kohlmünzer S (1995) Sesquiterpene lactones in Agrobacterium rhizogenes-transformed hairy root culture of Lactuca virosa. Phytochemistry 40:1139–1140
Kodoma M, Wada H, Otani H, Kohmoto K, Kimura Y (1998) 3,5-Di-O-caffeoylquinic acid, an infection-inhibiting factor from Pyrus pyrifolia induced by infection with Alternaria alternata. Phytochemistry 47:371–373
Leiss KA, Maltese F, Choi YH, Verpoorte R, Klinkhamer PGL (2009) Identification of chlorogenic acid as a resistance factor for thrips in Chrysanthemum. Plant Physiol 150:1567–1575
Li W–W, Barz W (2006) Structure and accumulation of phenolics in elicited Echinacea purpurea cell cultures. Planta Med 72:248–254
Liu C-Z, Abbasi BH, Gao M, Murch SJ, Saxena PK (2006) Caffeic acid derivatives production by hairy root cultures of Echinacea purpurea. J Agric Food Chem 54:8456–8460
Llorach R, Martínez-Sánchez A, Tomás-Barberán FA, Gil MI, Ferreres F (2008) Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem 108:1028–1038
McDougall B, King PJ, Wu B-W, Hostomsky Z, Reinecke MG, Robinson WE Jr (1998) Dicaffeoylquinic and dicaffeoyltartaric acids are selective inhibitors of human immunodeficiency virus type I integrase. Antimicrob Agents Chemother 42:140–146
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nicolle C, Carnat A, Fraisse D, Lamaison J-L, Rock E, Michel H, Amouroux P, Remesy C (2004) Characterisation and variation of antioxidant micronutrients in lettuce (Lactuca sativa folium). J Sci Food Agric 84:2061–2069
Ohnishi M, Morishita H, Iwahashi H, Toda S, Shirataki Y, Kimura M, Kido R (1994) Inhibitory effects of chlorogenic acids on linoleic acid peroxidation and haemolysis. Phytochemistry 36:579–583
Olmos A, Giner RM, Recio MC, Ríos JL, Gil-Benso R, Máñez S (2008) Interaction of dicaffeoylquinic derivatives with peroxynitrite and other reactive nitrogen species. Arch Biochem Biophys 475:66–71
Park KH, Park M, Choi SE, Jeong MS, Kwon JH, Oh MH, Choi HK, Seo SJ, Lee MW (2009) The anti-oxidative and anti-inflammatory effects of caffeoyl derivatives from the roots of Aconitum koreanum R. Raymond Biol Pharm Bull 32:2029–2033
Pauli GF, Poetsch F, Nahrstedt A (1998) Structure assignment of natural quinic acid derivatives using proton nuclear magnetic resonance techniques. Phytochem Anal 9:177–185
Pellati F, Benvenuti S, Magro L, Melegari M, Soragni F (2004) Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. J Pharm Biomed Anal 35:289–301
Ribas-Agustí A, Gratacós-Cubarsí M, Sárraga C, García-Regueiro J -A, Castellari M (2011). Analysis of eleven phenolic compounds including novel p-coumaroyl derivatives in lettuce (Lactuca sativa L.) by ultra-high-performance liquid chromatography with photodiode array and mass spectrometry detection. Phytochem Anal. doi:10.1002/pca.1318
Robinson WE Jr, Reinecke MG, Abdel-Malek S, Jia Q, Chow SA (1996) Inhibitors of HIV-1 replication that inhibit HIV integrase. Proc Natl Acad Sci 93:6326–6331
Spitaler R, Schlorhaufer PD, Ellmerer EP, Merfort I, Bortenschleger S, Stuppner H, Zidorn C (2006) Altitudinal variation of secondary metabolite profiles in flowering heads of Arnica montana cv. ARBO Phytochem 67:409–417
Stojakowska A, Kisiel W (2000) Neolignan glycosides from a cell suspension culture of Lactuca virosa. Pol J Chem 74:153–155
Stojakowska A, Malarz J (2000) Flavonoid production in transformed root cultures of Scutellaria baicalensis. J Plant Physiol 156:121–125
Stojakowska A, Malarz J, Kisiel W (1994) Sesquiterpene lactones in tissue culture of Lactuca virosa. Planta Med 60:93–94
Stojakowska A, Malarz J, Kisiel W (1999) Lactuca virosa L. (bitter lettuce): In vitro culture and production of sesquiterpene lactones. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. Medicinal and aromatic plants XI, vol 43. Springer-Verlag, Heidelberg, pp 261–273
Tamaki H, Robinson RW, Anderson JL, Stoewsand GS (1995) Sesquiterpene lactones in virus-resistant lettuce. J Agric Food Chem 43:6–8
Tamura H, Akioka T, Ueno K, Chujyo T, Okazaki K-I, King PJ, Robinson WE Jr (2006) Anti-human immunodeficiency virus activity of 3, 4, 5-tricaffeoylquinic acid in cultured cells of lettuce leaves. Mol Nutr Food Res 50:396–400
Wesołowska A, Nikiforuk A, Michalska K, Kisiel W, Chojnacka-Wójcik E (2006) Analgesic and sedative activities of lactucin and some lactucin-like guaianolides in mice. J Ethnopharmacol 107:254–258
Wu C-H, Murthy HN, Hahn E-J, Paek KY (2007) Enhanced production of caftaric acid, chlorogenic acid and cichoric acid in suspension cultures of Echinacea purpurea by the manipulation of incubation temperature and photoperiod. Biochem Eng J 36:301–303
Zha R-P, Xu W, Wang W-Y, Dong L, Wang Y-P (2007) Prevention of lipopolisaccharide-induced injury by 3,5-dicaffeoylquinic acid in endothelial cells. Acta Pharmacol Sin 28:1143–1148
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Lewak.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Stojakowska, A., Malarz, J., Szewczyk, A. et al. Caffeic acid derivatives from a hairy root culture of Lactuca virosa . Acta Physiol Plant 34, 291–298 (2012). https://doi.org/10.1007/s11738-011-0827-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-011-0827-4