Abstract
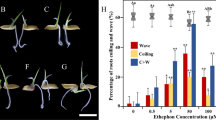
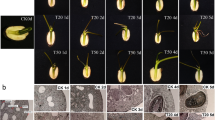
Adventitious roots (ARs) play an irreplaceable role in the uptake of water and nutrients due to under-developed principle root in plants. The process of ARs formation is affected by plant hormone. In this study, by employing High-Throughout Tag-sequencing Technique and ELISA method, we analyzed of the transcriptome and indole-3-acetic acid (IAA) content to monitor the changes of metabolism regulated by ethylene signaling in lotus. Exogenous application of ethephon (ethylene precursor) dramatically accelerated ARs development, and while restrained by 1-methylcyclopropene (1-MCP, the ethylene perception inhibitor), indicating the crucial role ethylene in ARs development. Transcriptomic analysis showed that both treatment of ethephon and 1-MCP dramatically altered the expression of numerous genes. In total, transcriptional expressions of 694 genes were induced and 554 genes were suppressed in ETH/CK0 stages compared with MCP/CK0 stages. Most of these up-regulated genes exhibited the one-three folds changes. In ETH/MCP libraries, we found nine and five genes involved in the metabolism or transcriptional responses to ethylene and IAA, and fourteen genes, which were considered to NAC, bHLH, AP2-EREBP, MYB, LOB, bHLH and bZIP families, respectively, exhibited an increase in transcriptional level. In addition, an enhanced mRNA levels of seven genes [1-aminocyclopropane-1-carboxylate oxidase (ACO), leucine-rich repeat receptor, pectinesterase, pyruvate decarboxylase, ethylene oxide synthase, respiratory burst oxidase homolog protein and xyloglucan endotransglucosylase] relevant to ARs formation were detected in was detected in ETH/MCP libraries. Furthermore, we found that IAA content was obviously decreased after applications were detected on ethephon and 1-MCP. However, the decreased IAA level in 1-MCP treatment was more pronounced than that in ethephon treatment, and kept a low level during the whole periods of ARs development. Taken together, our findings provided a comprehensive understanding of ethylene’s regulation during ARs formation in lotus seedlings.




Similar content being viewed by others
Availability of data and materials
All the raw tags related to ARs formation in responding to ethephon and 1-MCP treatment were deposited in NCBI database (BioProject: PRJNA451341; Accession:SAMN08968558 ~ SAMN08968558).
Abbreviations
- 1-MCP:
-
1-Methylcyclopropene
- IAA:
-
Indole-3-acetic acid
- ARs:
-
Adventitious roots
- ACC:
-
1-Aminocyclopropane-1-carboxylic acid
- ACO:
-
1-Aminocyclopropane-1-carboxylate oxidase
- DEGs:
-
Differentially expressed genes
References
Abeles FB, Morgan PW, Saltveit JME (1992) Ethylene in plant biology. Academic Press, San Diego
Ahkami A, Scholz U, Steuernagel B, Strickert M, Haensch KT, Druege U, Reinhardt D, Nouri E, Wiren NV, Franken P, Hajirezaei MR (2014) Comprehensive transcriptome analysis unravels the existence of crucial genes regulating primary metabolism during adventitious root formation in petunia hybrid. PLoS One 9:e100997
Bhosale R, Boudolf V, Cuevas F et al (2018) A spatiotemporal DNA endoploidy map of the arabidopsis root reveals roles for the endocycle in root development and stress adaptation. Plant Cell 30:2330–2351
Borgi W, Ghedira K, Chouchane N (2007) Anti inflammatory and analgesic activities of Zizyphus lotus root barks. Fitoterapia 78:16–19
Cheng LB, Li SY, Yin JJ, Li LJ, Chen XH (2013a) Genome-wide analysis of differentially expressed genes relevant to rhizome formation in lotus root (Nelumbo nucifera Gaertn.). Plos One 8:e67116
Cheng LB, Li SY, Xu XY, Hussain J, Yin JJ, Zhang Y (2013b) Identification of differentially expressed genes relevant to corm formation in Sagittaria trifolia. PLoS One 8:e54573
Cheng LB, Li SY, Chen SN, Wang Y, Yu MZ, Chen XH, Li LJ, Yin JJ (2016) Transcriptome analysis of gene expression during Chinese water chestnut storage organ formation. PLoS One 11:e0164223
Cheng LB, Jiang RZ, Yang JJ, Xu XY, Zeng HT, Li SY (2018) Transcriptome profiling reveals an IAA-regulated response to adventitious roots formation in lotus seedling. Zeitschrift fur Nat 25:30. https://doi.org/10.1515/znc-2017-0188
Christopher J, Christopher M, Jennings R, Jones S, Fletcher S, Borrell A (2013) QTL for root angle and number in a population developed from bread wheats (Triticum aestivum) with contrasting adaptation to water-limited environments. Theor Appl Genet 126:1563–1574
Clark DG, Gubrium EK, Barrett JE, Nell TA, Klee HJ (1999) Root formation in ethylene-insensitive plants. Plant Physiol 121:53–59
Da Costa CT, de Almeida MR, Ruedell CM, Schwambach J, Maraschin FS, Fett-Neto AG (2012) When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings. Front Plant Sci 4:133
Delarue M, Prinsen E, Onckelen VH, Caboche M, Bellini C (1998) Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. Plant J 14:603–611
Druege U, Franken P, Lischewski SA, Ahkami AH, Zerche S, Hause B (2014) Transcriptomic analysis reveals ethylene as stimulator and auxin as regulator of adventitious root formation in petunia cuttings. Frontiers in Plant Sci 5:494
Falasca G, Zaghi D, Possenti M, Altamura MM (2004) Adventitious root formation in Arabidopsis thaliana thin cell layers. Plant Cell Rep 23:17–25
Fiserova H, Mikusova Z, Klems M (2008) Estimation of ethylene production and 1-aminocyclopropane-1-carboxylic acid content in plants by means of gas chromatography. Plant Soil Environ 54:55–60
Fukaki H, Tasaka M (2009) Hormone interactions during lateral root formation. Plant Mol Biol 69:437–449
Ge ZGEL, Wang L (2012) Molecular mechanism of adventitious root formation in rice. Plant Growth Regul 68:325–331
Geiss G, Gutierrez L, Bellini C (2009) Adventitious root formation: new insights and perspectives. Annu. Plant Rev 37:127–156
Guo SY, Dai SJ, Singh PK et al (2018) A membrane-bound NAC-Like transcription factor OsNTL5 represses the flowering in Oryza sativa. Front plant sci 9:9
He XJ, Mu RL, Cao WH, Chen SY (2006) AtNAC2, a transcription factor downstream of ethylene and auxin signaling pathways, is involved in salt stress response and lateral root development. Plant J 44:903–916
Jiao K, Li X, Guo Y et al (2019) Regulation of compound leaf development in mungbean (Vigna radiata L.) by CUP-SHAPED COTYLEDON/NO APICAL MERISTEM (CUC/NAM) gene. Planta 249:765–774
Kim JS, Yamaguchi-Shinozaki K, Shinozaki K (2018) ER-anchored transcription factors bZIP17 and bZIP28 regulate root elongation. Plant Physiol. https://doi.org/10.1104/pp.17.01414
Kohli A, Sreenivasulu N, Lakshmanan P, Kumar PP (2013) The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep 32:945–957
Legué V, Rigal A, Bhalerao RP (2014) Adventitious root formation in tree species: involvement of transcription factors. Physiol Plant 151:192–198
Li SW, Leng Y, Feng L, Zeng XY (2014) Involvement of abscisic acid in regulation Antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean [Vigna radiata (L.)Wilczek] seedlings under cadmium stress. Environ Sci Pollut Res 21:525–537
Li L, Hou MJ, Cao L, Xia Y, Shen ZG, Hu ZB (2018) Glutathione S-transferases modulate Cu tolerance in Oryza sativa. Environ Exp Bot 155:313–320
Li X, Liu W, Zhuang L et al (2019) BIGGER ORGANS and ELEPHANT EAR-LIKE LEAF1 control organ size and floral organ internal asymmetry in pea. J Exp Bot 70:179–191
Liu HJ, Wang SF, Yu XB, Yu J, He XW, Zhang SL, Shou HX, Wu P (2005) ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 43:47–56
Liu RX, Chen SM, Jiang JF, Zhu L, Zheng C, Han S, Gu J, Sun J, Li HY, Wang HB, Song AP, Chen FD (2013) Proteomic changes in the base of chrysanthemum cuttings during adventitious root formation. BMC Genom 14:919
Ludwig-Muller J, Vertocnik A, Town CD (2005) Analysis of indole-3-butyric acid induce adventitious root formation on Arabidopsis stem segments. J Exp Bot 56:2095–2105
Mach J (2011) Unpureeing the tomato layers of information revealed by microdissection and high-throughput transcriptome sequencing. Plant Cell. 23:3868
Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14:589–597
Mensuali-Sodi A, Panizza M, Tognoni F (1995) Endogenous ethylene requirement for adventitious root induction and growth in tomato cotyledons and lavandin micro cuttings in vitro. Plant Growth Regul 17:205–212
Mergemann H, Sauter M (2000) Ethylene induces epidermal cell death at the site of adventitious root emergence in rice. Plant Physiol 124:609–614
Ming R, Van Buren R, Liu YL, Yang M, Han YP, Li LT (2013) Genome of the long-living sacred lotus (Nelumbo nucifera Gaertn.). Genome Biol 14:R41
Nag S, Saha K, Choudhuri MA (2001) Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J Plant Growth Regul 20:182–194
Negi S, Sukumar P, Liu X, Cohen JD, Muday GK (2010) Genetic dissection of the role of ethylene in regulating auxin-dependent lateral and adventitious root formation in tomato. Plant J 61:3–15
Pan RC, Wang JZ, Tian XS (2002) Influence of ethylene on adventitious root formation in mung bean hypocotyl cuttings. J Plant Growth Regul 36:135–139
Pence VC, Caruso JL (1987) Elisa determination of IAA using antibodies against ring-linked IAA. Phytochem 26:1251–1255
Pluss R, Jenny T, Meier H (1989) IAA-induced adventitious root formation in greenwood cuttings of Populus tremula and formation of 2-indolone-3-acetylaspartic acid, a new metabolite of exogenously applied indole-3-acetic aci. Physiol Plant 75:89–96
Qi XH, Xu XW, Lin XJ, Zhang WJ, Chen XH (2011) Identification of differentially expressed genes in cucumber (Cucumis sativus L.) root under waterlogging stress by digital gene expression profile. Genomics 99:160–168
Qu X, Cao B, Kang J et al (2019) Fine-tuning stomatal movement through small signaling peptides. Front Plant Sci 10:69
Rasmussen A, Hosseini SA, Hajirezaei MR, Druege U, Geelen D (2015) Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J Exp Bot 66:1437–1452
Rigal A, Yordanov YS, Perrone I, Karlberg A, Tisserant E, Bellini C (2012) The AINTEGUMENTA LIKE1 homeotic transcription factor PtAIL1 controls the formation of adventitious root primordia in poplar. Plant Physiol 160:1996–2006
Riov J, Yang SF (1989) Ethylene and auxin-ethylene interaction in adventitious root formation in mung bean (Vigna radiata) cuttings. J Plant Growth Regul 8:131–141
Rout GR (2006) Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Regul 48:111–117
Rovere D, Fattorini L, Angeli SD, Veloccia A, Falasca G, Altamura MM (2013) Auxin and cytokinin control formation of the quiescent centre in the adventitious root apex of Arabidopsis. Ann Bot 112:1395–1407
Sauer M, Balla J, Luschnig C, Wisniewska J, Reinöhl V, Friml J, Benková E (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20:2902–2911
Shen-Miller J (2002) Sacred lotus, the long-living fruits of China Antique. Seed Sci Res 12:131–143
Sieberer T, Leyser O (2006) Plant science-Auxin transport, but in which direction? Sci 312:858–860
Sorin CL, John D, Camus BI, Ljung K, Kowalczyk M, Geiss G, McKhann H, Garcion C, Vaucheret H, Sandberg G, Bellini C (2005) Auxin and light control of adventitious rooting in Arabidopsis Require ARGONAUTE1. Plant Cell 17:1343–1359
Steffens B, Wang JX, Sauter M (2006) Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223:604–612
Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19:2169–2185
Strader LC, Chen GL, Bartel B (2010) Ethylene directs auxin to control root cell expansion. Plant J 64:874–884
Taramino G, Sauer M Jr, Multani D, Niu XM, Sakai H (2007) The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J 50:649–659
Teale WD, Paponov IA, Palme K (2006) Auxin in action: signaling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7:847–859
Trupiano D, Yordanov Y, Regan S, Meilan R, Tschaplinski T, Scippa GS (2013) Identification, characterization of an AP2/ERF transcription factor that promotes adventitious, lateral root formation in Populus. Planta 238:271–282
Vanneste S, Friml J (2009) Auxin: a trigger for change in plant development. Cell 136:1005–1016
Visser E, Cohen JD, Barendse G, Blom C, Voesenek L (1996) An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded rumex palustrissm. Plant Physiol 112:1687–1692
Wang QQ, Liu F, Chen XS, Ma XJ, Zeng HQ, Yang ZM (2010) Transcriptome profiling of early developing cotton fiber by deep-sequencing reveals significantly differential expression of genes in a fuzzless/lintless mutant. Genomics 96:369–376
Wang FD, Li LB, Li HY, Liu LF, Zhang YH, Ga JW (2012) Transcriptome analysis of rosette and folding leaves in Chinese cabbage using high-throughput RNA sequencing. Genomics 99:299–307
Wang DJ, Yang CL, Dong L, Zhu JC, Wang JP, Zhang SF (2015) Comparative transcriptome analyses of drought-resistant and—Susceptible Brassica napus L. and development of EST-SSR markers by RNA-seq. J Plant Biol 58:259–269
Wang P, Yang CL, Chen H, Song CP, Zhang X, Wang DJ (2017) Transcriptomic basis for drought-resistance in Brassica napus L. Sci Rep 7:20
Wang ZQ, Yang CL, Chen H et al (2018) Multi-gene co-expression can improve comprehensive resistance to multiple abiotic stresses in Brassica napus L. Plant Sci 274:410–419
Xu M, Zhu L, Shou HX, Wu P (2005) A PIN1 Family Gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in Rice. Plant Cell Physiol 46:1674–1681
Zhang L, Shi X, Zhang Y et al (2019) CLE9 peptide-induced stomatal closure is mediated by abscisic acid, hydrogen peroxide, and nitric oxide in Arabidopsis thaliana. Plant, Cell Environ 42:1033–1044
Acknowledgments
This work was supported by Jiangsu Agriculture Science and Technology Innovation Fund (CX (18) 3066), and the modern agriculture of Yangzhou (YZ2017044). We extend our thanks to some members of BIG for their cooperation in obtaining the data during ARs formation of the lotus by RNA-seq technique. The authors also thank Edanz Group Ltd for their editorial assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Communicated by R. Aroca.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Libao, C., yuyan, H., Huiying, L. et al. Transcriptomic analysis reveals ethylene’s regulation involved in adventitious roots formation in lotus (Nelumbo nucifera Gaertn.). Acta Physiol Plant 41, 97 (2019). https://doi.org/10.1007/s11738-019-2895-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-019-2895-9