Abstract
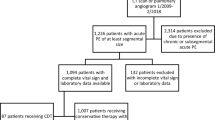
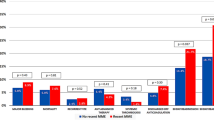
We sought to compare length-of-stay (LOS), total hospital costs, and readmissions among pulmonary embolism (PE) patients treated with rivaroxaban versus parenterally bridged warfarin. We identified adult PE (primary diagnostic code = 415.1x) patients in the Premier Database (11/2012-9/2015), and included those with ≥1 PE diagnostic test on days 0–2. Rivaroxaban users (allowing ≤2 days of prior parenteral therapy) were 1:1 propensity score matched to patients parenterally bridged to warfarin. LOS, total costs, and readmission for venous thromboembolism (VTE) or major bleeding within the same or subsequent 2 months were compared between cohorts. Separate analyses were performed in low-risk PE patients. Rivaroxaban use was associated with a 1.4-day [95 % confidence interval (CI) −1.47 to −1.28] shorter LOS, and $2322 (95 % CI −$2499 to −$2146) reduction in costs compared to parenterally bridged warfarin (p < 0.001 for both). There was no difference in readmission for VTE (1.5 versus 1.7 %) or major bleeding (0.3 versus 0.2 %) between the rivaroxaban and parenterally bridged warfarin cohorts (p ≥ 0.27 for both). Results were similar in low-risk patients (0.2–1.0 day and $251–$1751 reductions in LOS and costs, respectively, p ≤ 0.01 for all). In patients with PE, rivaroxaban was associated with reduced LOS and costs, without increased risk of readmission versus parenterally bridged warfarin. Similar results were observed in low-risk PE patients.
Similar content being viewed by others
References
HCUPnet, Healthcare Cost and Utilization Project (2013) Agency for Healthcare Research and Quality. http://hcupnet.ahrq.gov/. Accessed 28 June 2016
Kearon C, Akl EA, Ornelas J et al (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149:315–352
Coleman CI, Peacock WF, Weeda ER, Ashton V, Fermann GJ (2016) Outcomes related to variation in hospital pulmonary embolus observation stay utilization. Hosp Pract 27:1–5
EINSTEIN–PE Investigators, Büller HR, Prins MH et al (2012) Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med 366:1287–1297
van Bellen B, Bamber L, Correa de Carvalho F, Prins M, Wang M, Lensing AW (2014) Reduction in the length of stay with rivaroxaban as a single-drug regimen for the treatment of deep vein thrombosis and pulmonary embolism. Curr Med Res Opin 30:829–837
Cunningham A, Stein CM, Chung CP, Daugherty JR, Smalley WE, Ray WA (2011) An automated database case definition for serious bleeding related to oral anticoagulant use. Pharmacoepidemiol Drug Saf 20:560–566
Coleman CI, Kohn CG, Bunz TJ (2015) Derivation and validation of the In-hospital Mortality for Pulmonary embolism using Claims daTa (IMPACT) prediction rule. Curr Med Res Opin 31:1461–1468
Weeda ER, Kohn CG, Fermann GJ et al (2016) External validation of prognostic rules for early post-pulmonary embolism mortality: assessment of a claims-based and three clinical-based approaches. Thromb J 14:7
Piran S, Le gal G, Wells PS et al (2013) Outpatient treatment of symptomatic pulmonary embolism: a systematic review and meta-analysis. Thromb Res 132:515–519
Austin PC (2011) An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar Behav Res 46:399–424
Elixhauser A, Steiner C, Harris DR, Coffey RM (1998) Comorbidity measures for use with administrative data. Med Care 36:8–27
Bookhart BK, Haskell L, Bamber L, Wang M, Schein J, Mody SH (2014) Length of stay and economic consequences with rivaroxaban vs enoxaparin/vitamin K antagonist in patients with DVT and PE: findings from the North American EINSTEIN clinical trial program. J Med Econ 17:691–695
Roberts KM, Knight TB, Padilla-tolentino E, Murthy M, Peterson EJ (2015) Length of stay comparison between rivaroxaban and warfarin in the treatment of pulmonary embolism: results from a real-world observational cohort study. Thrombosis 2015:414–523
Weeda ER, Kohn CG, Peacock WF et al (2016) Rivaroxaban versus heparin bridging to warfarin in low-risk patients with pulmonary embolism. Pharmacotherapy. doi:10.1002/phar.1828 (Epub ahead of print)
Kohn CG, Mearns ES, Parker MW, Hernandez AV, Coleman CI (2015) Prognostic accuracy of clinical prediction rules for early post-pulmonary embolism all-cause mortality: a bivariate meta-analysis. Chest 147:1043–1062
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This analysis was sponsored by Janssen Scientific Affairs, LLC, Raritan, NJ, USA.
Conflict of interest
Dr. Coleman has received Grant funding and consultancy fees from Janssen Pharmaceuticals; Bayer Pharma AG and Boehringer-Ingelheim Pharmaceuticals, Inc. Mrs. Ashton and Drs. Crivera, Schein, and Wildgoose are employees of Janssen Scientific Affairs LLC. Dr. Peacock has received Grant funding and consultancy fees from Janssen Pharmaceuticals and Portola. Dr. Fermann has received Grant funding from Pfizer and is on the advisory board and speaker’s bureau for Janssen Pharmaceuticals. Dr. Baugh is a Roche Diagnostics Advisory Board member and received consulting fees from Janssen Pharmaceuticals. He also reports research support from Janssen Pharmaceuticals and Boehringer Ingelheim. Dr. Wells has received Grant funding from Bristol Myers Squib and Pfizer, is on the advisory board and has received speaker’s fees from Bayer Healthcare, has received consultancy fees from Janssen Pharmaceuticals, and served on a writing committee with Itreas. Dr. Weeda has no conflict of interest germane to this manuscript to report. No non-financial conflicts of interest exist for any of the authors.
Statements on human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required. It should also be noted that all data were de-identified; thus, our study was exempted from institutional review board oversight.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Weeda, E.R., Wells, P.S., Peacock, W.F. et al. Hospital length-of-stay and costs among pulmonary embolism patients treated with rivaroxaban versus parenteral bridging to warfarin. Intern Emerg Med 12, 311–318 (2017). https://doi.org/10.1007/s11739-016-1552-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11739-016-1552-1