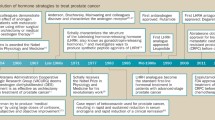
Opinion statement
Recent results of phase III randomized studies confirm that targeting the androgen receptor (AR)—through inhibition of androgen synthesis or through AR targeting directly—can improve survival for patients with metastatic castration-resistant prostate cancer (mCRPC), a condition previously considered to be “refractory” to further hormonal manipulation. These data validate in the clinical setting much of the scientific work of the previous decade that has demonstrated the extent of and mechanisms behind retained AR signaling in advanced prostate cancer. The convergence of these observations effectively changes the perspective with which androgen deprivation is utilized in prostate cancer, and forms the basis for further expansion of systemic therapy in the disease. In this review, the rationale for and clinical results with these new therapies will be discussed as will the future directions required to fully leverage these therapeutic modalities to the maximum clinical benefit for patients.

Similar content being viewed by others
References and Recommended Readings
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Huggins C, Hodges CV. Studies on prostatic cancer, effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7.
Crawford ED, Eisenberger MA, McLoed DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321:419–24.
Dijkman GA, Janknegt RA, De Reijke THM, et al. Long-term efficacy and safety of nilutamide plus castration in advanced stage prostate cancer, and the significance of early prostate specific antigen normalization. J Urol. 1997;158:160–3.
Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339:1036–42.
Edwards J, Krishna NS, Grigor KM, et al. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br J Cancer. 2003;89:552–6.
Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–27.
Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25.
Taplin ME, Bubley GJ, Ko YJ, et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59:2511–5.
Sun S, Sprenger CC, Vessella RL, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120:2715–30.
Guo Z, Yang X, Sun F, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69:2305–13.
Taylor CD, Elson P, Trump DL. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993;11:2167–72.
Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
Kantoff PW, Higano CS, Shore ND, et al: Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 363:411–22
de Bono JS, Oudard S, Ozguroglu M, et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 376:1147–54
de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
Cai C, Chen S, Ng P, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–13.
Pont A, Williams PL, Azhar S, et al. Ketoconazole blocks testosterone synthesis. Arch Intern Med. 1982;142:2137–40.
De Coster R, Caers I, Coene MC, et al. Effects of high dose ketoconazole therapy on the main plasma testicular and adrenal steroids in previously untreated prostatic cancer patients. Clin Endocrinol (Oxf). 1986;24:657–64.
Haidar S, Ehmer PB, Barassin S, et al. Effects of novel 17alpha-hydroxylase/C17, 20-lyase (P450 17, CYP 17) inhibitors on androgen biosynthesis in vitro and in vivo. J Steroid Biochem Mol Biol. 2003;84:555–62.
Small EJ, Baron AD, Fippin L, et al. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol. 1997;157:1204–7.
Small E, Halabi S, Dawson NA, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004;22:1025–33.
Barrie SE, Potter GA, Goddard PM, et al. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17-20 lyase). J Steroid Biochem Mol Biol. 1994;50:267–73.
Potter GA, Barrie SE, Jarman M, et al. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17,20-lyase): potential agents for the treatment of prostatic cancer. J Med Chem. 1995;38:2463–71.
Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71.
Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010;28:1481–8.
Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–8.
Matsunaga N, Kaku T, Itoh F, et al. C17,20-lyase inhibitors I. Structure-based de novo design and SAR study of C17,20-lyase inhibitors. Bioorg Med Chem. 2004;12:2251–73.
Dreicer R, Agus DB, MacVicar GR, et al. Safety, pharmacokinetics, and efficacy of TAK-700 in metastatic castration-resistant prostrate cancer: A phase I/II, open-label study. J Clin Oncol. 2010;28:15s.
Agus DB, Stadler WM, Shevrin D, et al. Safety, efficacy, and pharmacodynamics of the investigational agent TAK-700 in metastatic castration-resistant prostate cancer (mCRPC): updated data from a phase 1/2 study. Chicago: American Society of Clinical Oncology; 2011. Phase I/II study demonstrating the clinical efficacy of orteronel, including in a group of patients who did not receive prednisone. The rate of adverse events related to secondary mineralocorticoid excess was low, lending support to the hypothesis that orteronel may be administered without steroids. This remains to be seen.
Vasaitis T, Belosay A, Schayowitz A, et al. Androgen receptor inactivation contributes to antitumor efficacy of 17{alpha}-hydroxylase/17,20-lyase inhibitor 3beta-hydroxy-17-(1H-benzimidazole-1-yl)androsta-5,16-diene in prostate cancer. Mol Cancer Ther. 2008;7:2348–57.
Fowler JE, Pandey P, Seaver LE, et al. Prostate specific antigen after gonadal androgen withdrawal and deferred flutamide treatment. J Urol. 1995;154:448–53.
Small EJ, Srinivas S. The antiandrogen withdrawal syndrome: experience in a large cohort of unselected patients with advanced prostate cancer. Cancer. 1995;76:1428–34.
Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9.
Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–90.
Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375:1437–46.
Scher, HI: Effect of MDV3100 an androgen receptor signaling inhibitor (ARSI), on overal survival in patient with prostate cancer postdocetaxel: results from the phase III AFFIRM study. J Clin Oncol. 2012;30(suppl 5, abstr LBA1). After planned interim analysis at 520 deaths, the study was unblinded and patient on placebo were offered treatment with MDV3100 based on the significant improvement in overall survival in patient treated with MDV3100.
Sawyers CL: New Insights into the Prostate Cancer Genome and Therapeutic Implications, State of the Science Report. Presented at the 17th Annual Prostate Cancer Foundation Scientific Retreat, Washington D.C., 2010
Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69:16–22.
Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22.
Carver BS, Chapinski C, Wongvipat J, et al. Reciprocal Feedback Regulation of PI3K and Androgen Receptor Signaling in PTEN-Deficient Prostate Cancer. Cancer Cell. 2011;19:575–86.
Darshan MS, Loftus MS, Thadani-Mulero M, et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 2011;71:6019–29.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, W., Ryan, C.J. Androgen Receptor Directed Therapies in Castration-Resistant Metastatic Prostate Cancer. Curr. Treat. Options in Oncol. 13, 189–200 (2012). https://doi.org/10.1007/s11864-012-0188-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11864-012-0188-2