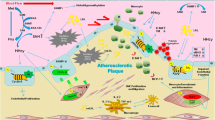
Abstract
The term epigenetics is usually used to describe inheritable changes in gene function which do not involve changes in the DNA sequence. These typically include non-coding RNAs, DNA methylation and histone modifications. Smoking and older age are recognised risk factors for peripheral artery diseases, such as occlusive lower limb artery disease and abdominal aortic aneurysm, and have been implicated in promoting epigenetic changes. This brief review describes studies that have associated epigenetic factors with peripheral artery diseases and investigations which have examined the effect of epigenetic modifications on the outcome of peripheral artery diseases in mouse models. Investigations have largely focused on microRNAs and have identified a number of circulating microRNAs associated with human peripheral artery diseases. Upregulating or antagonising a number of microRNAs has also been reported to limit aortic aneurysm development and hind limb ischemia in mouse models. The importance of DNA methylation and histone modifications in peripheral artery disease has been relatively little studied. Whether circulating microRNAs can be used to assist identification of patients with peripheral artery diseases and be modified in order to improve the outcome of peripheral artery disease will require further investigation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Krishna SM, Moxon JV, Golledge J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int J Mol Sci. 2015;16:11294–322.
Golledge J, Norman PE. Pathophysiology of abdominal aortic aneurysm relevant to improvements in patients’ management. Curr Opin Cardiol. 2009;24:532–8.
Golledge J. Lower-limb arterial disease. Lancet. 1997;350:1459–65.
Golledge J, Muller J, Daugherty A, Norman P. Abdominal aortic aneurysm: pathogenesis and implications for management. Arterioscler Thromb Vasc Biol. 2006;26:2605–13.
Norman PE, Davis TM, Le MT, Golledge J. Matrix biology of abdominal aortic aneurysms in diabetes: mechanisms underlying the negative association. Connect Tissue Res. 2007;48:125–31.
Golledge J, Norman PE. Atherosclerosis and abdominal aortic aneurysm: cause, response, or common risk factors? Arterioscler Thromb Vasc Biol. 2010;30:1075–7.
Khaleghi M, Isseh IN, Bailey KR, Kullo IJ. Family history as a risk factor for peripheral arterial disease. Am J Cardiol. 2014;114:928–32. This is one of the few studies to assess the association of family history with lower limb atherosclerosis associated occlusive disease and reported an odds ratio of 2.2.
Krishna SM, Dear AE, Norman PE, Golledge J. Genetic and epigenetic mechanisms and their possible role in abdominal aortic aneurysm. Atherosclerosis. 2010;212:16–29.
Krishna SM, Trollope AF, Golledge J. The relevance of epigenetics to occlusive cerebral and peripheral arterial disease. Clin Sci (Lond). 2015;128:537–58.
Krishna SM, Dear A, Craig JM, Norman PE, Golledge J. The potential role of homocysteine mediated DNA methylation and associated epigenetic changes in abdominal aortic aneurysm formation. Atherosclerosis. 2013;228:295–305.
Kloos W, Vogel B, Blessing E. MiRNAs in peripheral artery disease—something gripping this way comes. Vasa. 2014;43:163–70.
Adam M, Raaz U, Spin JM, Tsao PS. MicroRNAs in Abdominal Aortic Aneurysm. Curr Vasc Pharmacol. 2015;13:280–90.
Golledge J, Kuivaniemi H. Genetics of abdominal aortic aneurysm. Curr Opin Cardiol. 2013;28:290–6.
Spear R, Boytard L, Blervaque R, Chwastyniak M, Hot D, Vanhoutte J, et al. Adventitial tertiary lymphoid organs as potential source of microRNA biomarkers for abdominal aortic aneurysm. Int J Mol Sci. 2015;16:11276–93. This study used laser microdissection to look at MicroRNAs expressed in human AAA associated lymphoid tissue.
Cheuk BL, Cheng SW. Identification and characterization of microRNAs in vascular smooth muscle cells from patients with abdominal aortic aneurysms. J Vasc Surg. 2014;59:202–9.
Stather PW, Sylvius N, Sidloff DA, Dattani N, Verissimo A, Wild JB, et al. Identification of microRNAs associated with abdominal aortic aneurysms and peripheral arterial disease. Br J Surg. 2015;102:755–66. This study identifies a number of circulating MicroRNAs associated with AAA and lower limb occlusive artery disease.
Kin K, Miyagawa S, Fukushima S, Shirakawa Y, Torikai K, Shimamura K, et al. Tissue- and plasma-specific microRNA signatures for atherosclerotic abdominal aortic aneurysm. J Am Heart Assoc. 2012;1:e000745.
Biros E, Moran CS, Wang Y, Walker PJ, Cardinal J, Golledge J. microRNA profiling in patients with abdominal aortic aneurysms: the significance of miR-155. Clin Sci (Lond). 2014;126:795–803. miR-155 was identified as upregulated within aortic biopsies of human AAA and also serum of patients that had AAAs.
Pahl MC, Derr K, Gäbel G, Hinterseher I, Elmore JR, Schworer CM, et al. MicroRNA expression signature in human abdominal aortic aneurysms. BMC Med Genomics. 2012;5:25.
Li T, Cao H, Zhuang J, Wan J, Guan M, Yu B, et al. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin Chim Acta. 2011;412:66–70.
Zhang W, Shang T, Huang C, Yu T, Liu C, Qiao T, et al. Plasma microRNAs serve as potential biomarkers for abdominal aortic aneurysm. Clin Biochem. 2015;48:988–92. Three plasma microRNAs (miR-191-3p, miR-455-3p and miR-1281) were identified as having good sensitivity and specificity in identifying patients with AAA.
Dangwal S, Stratmann B, Bang C, Lorenzen JM, Kumarswamy R, Fiedler J, et al. Impairment of wound healing in patients with type 2 diabetes mellitus influences circulating microRNA patterns via inflammatory cytokines. Arterioscler Thromb Vasc Biol. 2015;35:1480–8.
Stather PW, Sylvius N, Wild JB, Choke E, Sayers RD, Bown MJ. Differential microRNA expression profiles in peripheral arterial disease. Circ Cardiovasc Genet. 2013;6:490–7. This study identified a number of microRNAs with good diagnostic ability for lower limb arterial occlusive disease as judged by receiver operator characteristic curves.
Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, et al. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun. 2014;5:5214. This study suggests that miR-24 inhibits AAA within a mouse model.
Zampetaki A, Attia R, Mayr U, Gomes RS, Phinikaridou A, Yin X, et al. Role of miR-195 in aortic aneurysmal disease. Circ Res. 2014;115:857–66.
Kim CW, Kumar S, Son DJ, Jang IH, Griendling KK, Jo H. Prevention of abdominal aortic aneurysm by anti-microRNA-712 or anti-microRNA-205 in angiotensin II-infused mice. Arterioscler Thromb Vasc Biol. 2014;34:1412–21.
Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, et al. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med. 2012;4:122ra22.
Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, et al. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest. 2012;122:497–506. This study confirms that miR-29b limits AAA within multiple mouse models.
Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, et al. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res. 2011;109:1115–9.
Merk DR, Chin JT, Dake BA, Maegdefessel L, Miller MO, Kimura N, et al. miR-29b participates in early aneurysm development in Marfan syndrome. Circ Res. 2012;110:312–24.
Lei Z, van Mil A, Brandt MM, Grundmann S, Hoefer I, Smits M, et al. MicroRNA-132/212 family enhances arteriogenesis after hindlimb ischaemia through modulation of the Ras-MAPK pathway. J Cell Mol Med. 2015;19:1994–2005.
Pankratz F, Bemtgen X, Zeiser R, Leonhardt F, Kreuzaler S, Hilgendorf I, et al. MicroRNA-155 exerts cell-specific antiangiogenic but proarteriogenic effects during adaptive neovascularization. Circulation. 2015;131:1575–89.
Welten SM, Bastiaansen AJ, de Jong RC, de Vries MR, Peters EA, Boonstra MC, et al. Inhibition of 14q32 microRNAs miR-329, miR-487b, miR-494, and miR-495 increases neovascularization and blood flow recovery after ischemia. Circ Res. 2014;115:696–708. This study suggests that gene silencing oligonucleotides targeted on 14q32 expressed microRNAs stimulate arteriogenesis in a mouse model of hind limb ischemia.
Endo-Takahashi Y, Negishi Y, Nakamura A, Ukai S, Ooaku K, Oda Y, et al. Systemic delivery of miR-126 by miRNA-loaded Bubble liposomes for the treatment of hindlimb ischemia. Sci Rep. 2014;4:3883.
Hazarika S, Farber CR, Dokun AO, Pitsillides AN, Wang T, Lye RJ, et al. MicroRNA-93 controls perfusion recovery after hindlimb ischemia by modulating expression of multiple genes in the cell cycle pathway. Circulation. 2013;127:1818–28.
Semo J, Sharir R, Afek A, Avivi C, Barshack I, Maysel-Auslender S, et al. The 106b∼25 microRNA cluster is essential for neovascularization after hindlimb ischaemia in mice. Eur Heart J. 2014;35:3212–23.
Yin KJ, Olsen K, Hamblin M, Zhang J, Schwendeman SP, Chen YE. Vascular endothelial cell-specific microRNA-15a inhibits angiogenesis in hindlimb ischemia. J Biol Chem. 2012;287:27055–64.
Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–3.
van Solingen C, de Boer HC, Bijkerk R, Monge M, van Oeveren-Rietdijk AM, Seghers L, et al. MicroRNA-126 modulates endothelial SDF-1 expression and mobilization of Sca-1(+)/Lin(−) progenitor cells in ischaemia. Cardiovasc Res. 2011;92:449–55.
van Solingen C, Seghers L, Bijkerk R, Duijs JM, Roeten MK, van Oeveren-Rietdijk AM, et al. Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med. 2009;13:1577–85.
Krishna SM, Omer SM, Golledge J. Evaluation of the clinical relevance and limitations of current preclinical models of peripheral artery disease. Clin Sci. 2016;130:127–50.
Ryer EJ, Ronning KE, Erdman R, Schworer CM, Elmore JR, Peeler TC, et al. The potential role of DNA methylation in abdominal aortic aneurysms. Int J Mol Sci. 2015;16:11259–75. This study looks as the association of DNA methylation patterns with AAA.
Hu W, Wang M, Yin H, Yao C, He Q, Yin L, et al. MicroRNA-1298 is regulated by DNA methylation and affects vascular smooth muscle cell function by targeting connexin 43. Cardiovasc Res. 2015;107(4):534–45.
Vinh A, Gaspari TA, Liu HB, Dousha LF, Widdop RE, Dear AE. A novel histone deacetylase inhibitor reduces abdominal aortic aneurysm formation in angiotensin II-infused apolipoprotein E-deficient mice. J Vasc Res. 2008;45:143–52.
Golledge J, Norman PE. Current status of medical management for abdominal aortic aneurysm. Atherosclerosis. 2011;217:57–63.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jonathan Golledge declares that he works for James Cook University & The Townsville Hospital and declares grant money to his institution from NHMRC, Queensland Government.
Erik Biros, John Bingley, Vikram Iyer, and Smriti M Krishna declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Genetics
Rights and permissions
About this article
Cite this article
Golledge, J., Biros, E., Bingley, J. et al. Epigenetics and Peripheral Artery Disease. Curr Atheroscler Rep 18, 15 (2016). https://doi.org/10.1007/s11883-016-0567-4
Published:
DOI: https://doi.org/10.1007/s11883-016-0567-4