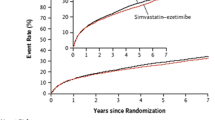
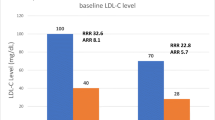Abstract
Over the past three decades, statins have become first-line treatment for reducing LDL cholesterol (LDL-C) and cardiovascular disease (CVD). They have provided a clear, robust, and reproducible relationship between the absolute LDL-C reduction and the decrease in CVD; every 1 mmol/L (∼40 mg/dL) in LDL-C reduction results in a 22 % decrease in CVD events. This relationship has recently been extended to reduction in LDL-C with a non-statin, ezetimibe, on top of statin therapy, further consolidating LDL-C as the cornerstone in CVD risk reduction. Despite these two effective and safe LDL-C-lowering drugs, there remains a need for additional drugs to reduce LDL-C, the focus of this review which covers agents which produce sufficient LDL-C reduction to potentially help address this unmet need and are either recently approved or currently in clinical trials.




Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344(8934):1383-1389.
Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and recurrent events trial investigators. N Engl J Med. 1996;335:1001–9.
Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333:1301–7.
Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22.
Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015. doi:10.1056/NEJMa1410489. Important trial which is first to demonstrate additional LDL-C lowering with non-statin added to statin resulted in further CVD risk reduction compared to statin alone.
Davidson MH, Dillon MA, Gordon B, et al. Colesevelam hydrochloride (cholestagel): a new, potent bile acid sequestrant associated with a low incidence of gastrointestinal side effects. Arch Intern Med. 1999;159(16):1893–900.
Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–74.
Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–67. HPS2-THRIVE.
Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–12.
Lambert G, Sjouke B, Choque B, et al. The PCSK9 decade. J Lipid Res. 2012;53:2515–24.
Stein EA, Raal FJ. Reduction of low density lipoprotein cholesterol by monoclonal antibody inhibition of PCSK9. Ann Rev Med. 2014;65:417–31.
Cohen JC, Boerwinkle E, Mosley Jr TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
Lagace TA, Curtis DE, Garuti R, et al. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest. 2006;116:2995–3005. Key finding in the PCSK9 relationship to high LDL-C in that it was plasma PCSK9 that resulted in LDL-C elevation thus allowing development of monoclonal antibodies to PCSK9.
Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Engl J Med. 2012;366:1108–83. First proof of concept in man that inhibition of PCSK9 would lower LDL-C in non-FH and FH patients.
Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888–98.
Stein EA, Raal FJ. New therapies for reducing low-density lipoprotein cholesterol. Endocrinol Metab Clin N Am. 2014;43:1007–33. Good review of drugs in development that lower LDL-C.
McKenney JM, Koren MJ, Kereiakes DJ, et al. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–53.
Roth EM, McKenney JM, Hanotin C, et al. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–900.
Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the gauss-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541–8. Large trial of statin adverse patients unable to tolerate at least 2 statins, or minimum doses of statins.
Moriarty PM, Jacobson TA, Bruckert E, et al. Efficacy and safety of alirocumab, a monoclonal antibody to PCSK9, in statin-intolerant patients: design and rationale of ODYSSEY ALTERNATIVE, a randomized phase 3 trial. J Clin Lipidol. 2014;8(6):554–61.
Stein EA, Gipe D, Bergeron J, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36.
Raal FJ, Scott R, Somaratne R, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia. The reduction of LDL-C with PSCK9 inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–17.
Raal FJ, Stein EA, Dufour R, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): results of a randomised double-blind, placebo controlled trial. Lancet. 2015;385:331–40. First trial in HeFH to assess genetic abnormalities causing FH and relationship to LDL-C response to evolocumab and demonstrating underlying defect was not related to response.
Stein EA, Honarpour N, Wasserman SM, Zu F, Scott R, Raal FJ. Effect of the proprotein convertase subtilisin/kexin 9 monoclonal antibody, AMG 145, in homozygous familial hypercholesterolemia. Circulation. 2013;128:2113–20. Proof-of-concept trial demonstrating PCSK9 inhibition would be effective in reducing LDL-C in HoFH.
Raal FJ, Honarpour N, Blom D, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): results of a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:341–50. Critical phase 3 trial of evolocumab in HoFH demonstrating response was related to underlying LDL receptor activity.
Sabatine MC, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9. First trial with PCSK9 monoclonal antibody to show in predefined exploratory analysis that CVD events are reduced.
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99. Confirmatory post hoc analysis with alirocumab to show reduction in CVD events with PCSK9 monoclonal antibody.
Ballantyne CM, Neutel J, Cropp A, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomised, placebo-controlled, dose-ranging study in statin treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115:1212–21.
Mitchell T, Chao G, Sitkoffv D. Pharmacologic profile of the adnectin BMS-962476, a small protein biologic alternative to PCSK9 antibodies for LDL lowering. J Pharmacol Exp Ther. 2014;350(2):412–24.
Stein EA, Kasichayanula S, Turner T, et al. LDL cholesterol reduction with BMS-962476, an adnectin inhibitor of PCSK9: results of a single ascending dose study. J Am Coll Cardiol. 2014;63(12 Suppl):A172. doi:10.1016/S0735-1097(14)61372-3.
Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res. 2007;48:763–7.
Fitzgerald K, Frank-Kamenetsky M, Shulga-Morskaya S, et al. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–8.
http://www.alnylam.com/web/assets/ALNY-ESC-GalNAc-siRNA-TIDES-May2014-Capella.pdf [accessed 9 June 2015]
Thompson A, Di Angelantonio E, Sarwar N, et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 2008;299:2777–88.
Krishna R, Anderson MS, Bergman AJ, et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet. 2007;370:1907–14.
Nicholls SJ, Brewer HB, Kastelein JJP, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA. 2011;306:2099–109.
A study of evacetrapib in high-risk vascular disease (ACCELERATE) http://clinicaltrials.gov/show/NCT01687998
REVEAL: Randomized EValuation of the Effects of Anacetrapib Through Lipid-modification. http://clinicaltrials.gov/show/NCT01252953
Bloomfield D, Carlson GL, Sapre A, et al. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am Heart J. 2009;157(2):352–60.
Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–15.
Kastelein JJP, Besseling J, Shah S, et al. Anacetrapib as lipid-modifying therapy in patients with heterozygous familial hypercholesterolaemia (REALIZE): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet. 2015;385:2153–61.
Hovingh GK, Kastelein JJP, van Deventer SJH, et al. Cholesterol ester transfer protein inhibition by TA-8995 in patients with mild dyslipidaemia (TULIP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet. 2015;386:452–60.
Pinkosky SL, Filippov S, Srivastava RA, et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J Lipid Res. 2013;54:134–51.
Goldberg R. Targeting low-density lipoprotein and dysmetabolism in type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34:477–8.
Filippov S, Pinkosky SL, Newton RS. LDL-cholesterol reduction in patients with hypercholesterolemia by modulation of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase. Curr Opin Lipidol. 2014;25(4):309–15.
Gutierrez MJ, Rosenberg NL, MacDougall DE, et al. Efficacy and safety of ETC-1002, a novel investigational low-density lipoprotein-cholesterol-lowering therapy for the treatment of patients with hypercholesterolemia and type 2 diabetes mellitus. Arterioscler Thromb Vasc Biol. 2014;34:676–83.
Rader DJ, Kastelein JJP. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014;129:1022–32.
Kastelein JJP, Wedel MK, Baker BF, et al. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006;114:1729–35.
Raal FJ, Santos RD, Blom DJ, et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:998–1006.
Wetterau JR, Aggerbeck LP, Bouma ME, et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992;258:999–1001.
Stein EA, Isaacsohn JL, Mazzu A, Ziegler R. Effect of BAY 13-9952, a microsomal triglyceride transfer protein inhibitor on lipids and lipoproteins in dyslipoproteinemic patients. Circulation 1999;100(18,Suppl 1) :Abst 1342
52.Farnier, M., E. Stein, S. Megnien, L. et al Efficacy and safety of implitapide, a microsomal triglyceride transfer protein inhibitor in patients with primary hypercholesterolemia. Abstract Book of the XIV International Symposium on Drugs Affecting Lipid Metabolism in New York, 2001
Implitapide in patients with homozygous familial hypercholesterolemia (HoFH) on maximal concurrent lipid-lowering therapy https://clinicaltrials.gov/ct2/show/NCT00079846 [accessed June 9, 2015]
Cuchel M, Meagher EA, du Toit TH, et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013;381:40–6.
Efficacy and safety of lomitapide in Japanese patients with HoFH on concurrent lipid-lowering therapy http://clinicaltrials.gov/ct2/show/record/NCT02173158 [accessed June 9, 2015]
Bays HE, Schwartz S, Littlejohn TIII, et al. MBX-8025, a novel peroxisome proliferator receptor-6 agonist: lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin. J Clin Endocrinol Metab. 2011;96:2889–97.
http://ir.cymabay.com/press-releases/detail/244/cymabay-therapeutics-announces-preclinical-data-demonstrating-the-potential-of-mbx-8025-to-treat-homozygous-familial-hypercholesterolemia [accessed June 9, 2015]
Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme a synthetases in metabolic control. Curr Opin Lipidol. 2010;21:212–7.
Bisgaier CL, Essenburg AD, Barnett BC, et al. A novel compound that elevates high density lipoprotein and activates the peroxisome proliferator activated receptor. J Lipid Res. 1998;39:17–30.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Evan A. Stein has received honoraria for consulting on the development of PCSK9 inhibitors from Amgen, Regeneron/Sanofi, Genentech/Roche, and BMS/Adnectin. He is a co-inventor of a use patent owned by Amgen for the use of PCSK9 monoclonal antibodies in homozygous familial hypercholesterolemia for which he receives no financial compensation. He also sits on the scientific advisory board for Catabasis, CymaBay, and Gemphire and has been a consultant for AstraZeneca.
Frederick J. Raal reports grants from the University of Witwatersrand, has received consulting fees from Amgen and Sanofi related to PCSK9 inhibitors and from Genzyme related to apolipoprotein B inhibitors, and has received personal fees and non-financial support from AstraZeneca, Pfizer, and Merck.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Lipid Abnormalities and Cardiovascular Prevention
Rights and permissions
About this article
Cite this article
Stein, E.A., Raal, F.J. Lipid-Lowering Drug Therapy for CVD Prevention: Looking into the Future. Curr Cardiol Rep 17, 104 (2015). https://doi.org/10.1007/s11886-015-0659-8
Published:
DOI: https://doi.org/10.1007/s11886-015-0659-8