Abstract
Purpose of Review
Long-COVID syndrome is a multi-organ disorder that persists beyond 12 weeks post-acute SARS-CoV-2 infection (COVID-19). Here, we provide a definition for this syndrome and discuss neuro-cardiology involvement due to the effects of (1) angiotensin-converting enzyme 2 receptors (the entry points for the virus), (2) inflammation, and (3) oxidative stress (the resultant effects of the virus).
Recent Findings
These effects may produce a spectrum of cardio-neuro effects (e.g., myocardial injury, primary arrhythmia, and cardiac symptoms due to autonomic dysfunction) which may affect all systems of the body. We discuss the symptoms and suggest therapies that target the underlying autonomic dysfunction to relieve the symptoms rather than merely treating symptoms. In addition to treating the autonomic dysfunction, the therapy also treats chronic inflammation and oxidative stress. Together with a full noninvasive cardiac workup, a full assessment of the autonomic nervous system, specifying parasympathetic and sympathetic (P&S) activity, both at rest and in response to challenges, is recommended. Cardiac symptoms must be treated directly. Cardiac treatment is often facilitated by treating the P&S dysfunction. Cardiac symptoms of dyspnea, chest pain, and palpitations, for example, need to be assessed objectively to differentiate cardiac from neural (autonomic) etiology.
Summary
Long-term myocardial injury commonly involves P&S dysfunction. P&S assessment usually connects symptoms of Long-COVID to the documented autonomic dysfunction(s).
Similar content being viewed by others
Introduction
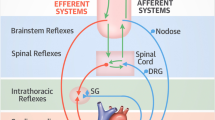
SARS-CoV-2 infection (COVID-19) is a major pandemic that is worldwide and itself is causing significant mortality and morbidity [1,2,3,4]. A subset of patients have presented with lingering, persistent, or prolonged symptoms for weeks or months afterwards, regardless of the severity of COVID infection [5,6,7,8,9]. This lingering condition has been labeled “post-acute sequelae of SARS-CoV-2 infection” syndrome or simply the “post-acute COVID-19 syndrome” or “long-COVID-19” or just “Long-COVID” or “long haulers COVID-19” or simply “long haulers” or “post-COVID syndrome” [5, 10]. This has extended the significant worldwide morbidity from the COVID-19 pandemic. It is estimated that 43% of patients who tested positive for SARS-COVID-19 will remain ill beyond 3 weeks [11], and this percentage may continue to rise. This is the subset that constitutes the Long-COVID syndrome. This does not include those who are not confirmed with acute COVID-19 that present with Long-COVID. Myocarditis is a common result of viral infection usually caused by oxidative stress due to the virus’ attack on the mitochondria in the heart muscle cell [12]. Oxidative stress also has a significant effect on the nervous system given that all nerves contain some of the highest amounts of mitochondria of all cells in the body. Oxidative stress produced in the mitochondria and cytosol of the heart, brain, and nervous system cells contributes to dysfunction and aging of the organs [13, 14]. The Cytokine storm involved in COVID-19 infections is a source of oxidative stress [15,16,17,18,19,20] (see Fig. 1 [21••]), and there are over 1200 references (circa. 2022) relating oxidative stress to parasympathetic and sympathetic (P&S) dysfunction [22,23,24]. Cardiac injury and primary arrhythmia may occur long-term in Long-COVID patients, but in our experience, these patients comprise a very small percentage of the Long-COVID population. The majority of Long-COVID patients with lingering cardio-neuro symptoms and disability present with P&S dysfunction(s) [25••].
The cytokine storm involved in COVID-19 infections is a source of oxidative stress. Viruses and traumas (mental or physical) in general may lead to oxidative stress, which may lead to parasympathetic or sympathetic dysfunction(s), known as dysautonomias (adapted from Rasa et al. [21••])
This prolonged post-COVID phase, with morbidity and ongoing symptoms, creates significant burden to the patient and to the healthcare system and is not completely understood. Not just quality of life, including mental and cognitive health, but employment and productivity issues become paramount when the acute, the subacute, and the chronic phases of COVID-19 occur [18]. The recovery from COVID-19 usually occurs at 7 to 10 days after the onset of symptoms in mild disease but could take 6 weeks or more in severe or critical cases. Laboratory abnormalities may be present and include low lymphocyte counts and elevated inflammatory markers (e.g., sedimentation rate, C-reactive protein, ferritin, interleukin 1 and 6, and tumor necrosis factor) [26,27,28,29,30,31,32,33,34,35,36]. Coagulation system abnormalities may occur [5, 26, 30, 32, 36,37,38]. Clots may form in the acute phase as well as in the subacute phase, especially if there is a history of thrombus formation. As suggested in Fig. 1, the symptoms of Long-COVID may be traced to P&S dysfunction and oxidative stress due to viral infection, including COVID-19 and other sources.
Defining Long-COVID
What exactly is Long-COVID syndrome? Long-COVID or post-COVID-19 is an umbrella term that refers to symptoms persisting past the initial phase. There are many definitions that have been offered [26, 28, 32, 34, 36]. Long-COVID has recently been defined as “the condition that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19, with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis” [39]. Likewise, there now exists an International Classification of Diseases, Tenth Revision (ICD-10) code corresponding to Long-COVID condition—U09.9 [40]. Basically, there are individuals who do not completely recover over a period of weeks, usually 2–3 weeks [41]. Since COVID-19 is a novel disease, there is still no consensus of the definition of Long-COVID symptoms. A systematic review [42], documented 20% of the reports of long-term COVID symptoms involved abnormal lung function, 24% involved neurological complaints and olfactory dysfunction, and 55% on specific widespread symptoms, mainly chronic fatigue and pain. The World Health Organization (WHO) developed a clinical case definition of Long-COVID by Delphi methodology that included 12 domains [43]. However, the understanding of this definition has been going through changes as new evidence emerges, and we are gaining a better understanding of the consequences of COVID-19 and its mutations. Usually, three or more months past the acute COVID-19 infection, symptoms that last for at least 2 months and cannot be explained by alternate diagnoses may fit this definition. These symptoms include fatigue, shortness of breath, cognitive dysfunction, and symptoms that affect the functional capacity of patients with daily living and productivity [41]. Symptoms may fluctuate, flare up, or relapse over time, adversely affecting multiple organ systems [26, 28, 30, 32, 34].
We propose that the delay between surviving the acute COVID infection and the onset of the Long-COVID symptoms is a function of the P&S nervous systems. The P&S nervous systems function together to coordinate and control organs and organ systems to maintain normal organ function, even when the two nervous systems are dysfunctional. Prolonged P&S dysfunction, once severe enough, then leads to poor organ control and then symptoms. This process may take up to 3 months, faster if there were prior comorbidities, including age. This is the basis for our claims that Long-COVID is a combination of both parasympathetic dysfunction(s) and sympathetic dysfunction(s). In our experience, the prolonged severe immune responses to COVID-19 seems to cause prolonged excessive parasympathetic responses, leading to secondary, prolonged, excessive beta-adrenergic (sympathetic) responses which prolongs and exaggerates heart rate, blood pressure, histaminergic, inflammatory, pain, and anxiety responses. The parasympathetic excess may also lead to both upper and lower GI symptoms. The oxidative stress of the acute COVID-19 infection also causes oxidative stress which often leads to alpha-adrenergic (sympathetic) dysfunction which leads to orthostatic dysfunction and poor coronary and cerebral perfusion and the perfusion of the anatomy in between causing many of the rest of the symptoms of Long-COVID. Long-COVID may directly affect the lungs, heart, nervous system, kidneys, and pancreas. Unfortunately, the lack of a standardized definition for Long-COVID syndrome presents obstacles for researchers in studying the condition with controlled studies and arriving at a precise diagnosis and treatment algorithms. In addition, many patients with Long-COVID syndrome require rehospitalization especially those with comorbidities, such as cardiovascular disease, diabetes mellitus, obesity, cancer, and kidney disease [5, 26, 41].
Data suggests that the evolution of Long-COVID syndrome is driven by cytokines produced in the body from inflammation [44, 45] which are often generated in response to viral infections and lead to oxidative stress. Post-infection olfactory dysfunction occurs in over 60% of those with acute SARS-CoV-2 infection, even in asymptomatic infection, and this represents an important frequent symptom of Long-COVID syndrome [5]. Also, loss of taste has been seen. Many patients are not diagnosed with Long-COVID syndrome, when in reality their taste or smelling remains impaired as the only symptom. Meta-analysis has shown the prevalence of Long-COVID manifestations [37, 42]. Fatigue and muscle weakness are by far the major symptoms followed by dyspnea and then pain and discomfort. Then there is anxiety and depression and impaired concentration. Insomnia and sleep disorders present with lower frequency. Interestingly, alopecia has been described as occurring. Chronic cough may persist, and arthralgias and myalgias may be present. Chest pain, cognitive impairment, dizziness, and headache are also symptoms but less common than the ones above. Persistent sore throat, palpitations, lack of smell, diarrhea, vomiting, fever, blurry vision, lack of taste, nasal congestion, anorexia, nausea, ringing in the ears, and rash may be present but at a much lower prevalence. These symptoms may be associated with parasympathetic or sympathetic dysfunction [41].
Our proposal that parasympathetic or sympathetic (autonomic) dysfunction underlies most of the disabilities associated with Long-COVID is based on questionnaires for autonomic symptoms (e.g., COMPASS 31). Two autonomic clusters in the Long-COVID syndrome have been identified [32, 46]. Impaired visual activity and blurry vision were more frequently registered during the acute phase in patients, while depression, chills, weakness, diarrhea, musculoskeletal, palpitations, tachycardia, dryness, cognitive dysfunction, headache, dizziness, and tinnitus were more frequently observed in the Long-COVID second cluster. The levels of antibodies to the SARS virus were not different between two clusters. This has given us some insight into one of the main mechanisms of Long-COVID syndrome, mainly autonomic dysfunction, which is an imbalance between the parasympathetic and sympathetic nervous systems. It is believed that a chronic inflammatory process, or autoimmune, or even a hormonal imbalance as a consequence of alterations in the hypothalamus–pituitary–adrenal axis may also be operative in conjunction with this acquired autonomic dysfunction with constitutional symptoms as described above [41].
The term Long-COVID syndrome was first used by Perego [47] and social media to describe symptoms weeks or months after the initial SARS-CoV2 infection. The term “long haulers” was used by Watson and by Yong [47,48,49]. There may be persistence of one or more symptoms of acute COVID or appearance of new symptoms, and they may be relapsing and remitting; therefore showing the variable presentation of Long-COVID syndrome. The majority of people with Long-COVID syndrome are polymerase chain reaction (PCR) negative including any microbiological recovery. Many people continue to see infectious disease doctors and seek out other alternative infection diagnoses when serology is continuously negative [41]. Others have defined Long-COVID as the time lag between the microbiologic recovery and clinical recovery [50]. The majority of those with Long-COVID syndromes have biochemical and radiological recovery. Others have postulated that there are two stages of Long-COVID syndrome: (1) symptoms that extend beyond 3 weeks but less than 12 weeks, which is more of a subacute phase, and (2) chronic COVID symptoms that extend beyond 12 months [51]. An interesting diagram and timeline (see Fig. 2 [51]) have been postulated, which shows that short COVID will generally last less than 3 weeks from the onset of symptoms. Post-acute COVID or subacute COVID will last from the onset of symptoms approximately up to 10–12 weeks, and chronic COVID will last from the onset of symptoms beyond 12 weeks. It would make sense to group the post-acute or subacute COVID, which lasts from up to 10–12 weeks, and then chronic COVID, which lasts more than 12 weeks as Long-COVID syndromes. In this review, however, we are more concerned with those symptoms that last more than 12 weeks, the true long- or prolonged COVID syndrome [41].
Classification of Long-COVID (reprinted from Raveendran AV et al. Diabetes Metab Syndr. 2021 May to Jun;15(3):869–875, with permission from Elsevier) [51]
Sometimes individuals are fairly asymptomatic [52], and by the time they develop Long-COVID symptoms, we do not know when the initial infection occurred or if it was COVID-19. With individuals who were symptomatic but not tested, it is more likely that this is a Long-COVID syndrome. The problem is not only with those patients who have persistent symptoms who have never tested positive for COVID-19, but with those who had upper respiratory tract infections with a negative COVID test and then developed prolonged symptoms. The question must be asked, “did they have a false-negative test performed too early or too late in the disease course?” Antibodies are unreliable as up to 20% patients do not seroconvert, and antibody levels decrease over time and by 3 months oftentimes are not measurable. Not only is the morbidity important with Long-COVID syndrome, but the economic cost to society, as 1/3 of the people in one survey did not return to their job for up to 3 weeks after being COVID-positive and a similar number of home makers have productivity difficulties. This is more common in people with pre-existing comorbidities and older patients. Not returning to work and productivity difficulties are often associated with obesity and psychiatric disease. In addition, given the similarity between Long-COVID syndrome and autonomic dysfunction and the ability of psychosocial stress to trigger symptoms of autonomic dysfunction further blur the distinction between Long-COVID and autonomic dysfunction [41].
The risk of COVID-19 is twice as common in women as it is in men [26]; thereby, potentially, doubling the risk of Long-COVID for women. Patients who develop Long-COVID syndrome are 4 years older than those who did not [53]. Given that age is associated with increased P&S dysfunction and symptoms, whether age is truly the primary factor is still a question. Patients with five or more symptoms in the acute phase of illness have a higher likelihood of developing a Long-COVID syndrome [41]. We have empirically found that obesity and increased body mass index are an extremely important risk factor as is female gender [41]. Symptoms that various studies report to be predictors of Long-COVID syndrome include diarrhea, anosmia, dyspnea, pleurisy, skin sensitivity, and an A blood type [54]. One study showed that a lower SARS-CoV-2 IgG titer at the beginning of the observation period was associated with a higher frequency of Long-COVID syndrome [55]. Also, the severity of acute COVID-19 suggests that the recovering critically ill patient commonly experiences long-lasting mental health issues that include depression, anxiety, post-traumatic stress, memory disorders, attention deficit disorders, and ongoing brain fog. This is controversial as others have shown that acute disease was not associated with Long-COVID syndrome. All of this negativity speaks to a heterogenous presentation of Long-COVID syndrome with risk factors including its timeline [41].
Even if the virus is cleared, there were high neutralized antibody titers that suggest that the immune system could continue to be overactive and induce this syndrome. Therefore, the question of autoimmunity is raised. This is especially true since viral shedding has shown that persistent fragments of viral genes and body secretions may cause hyperimmune responses, and this may explain some of the persistent symptoms in Long-COVID syndrome. Lingering autonomic dysfunction also presents with autoimmune-like symptoms [41]. As proposed above and demonstrated elsewhere [56, 57], prolonged, excessive parasympathetic activity may prolong and exaggerate immune responses, even after the infecting pathogen is eliminated, turning the body’s immune cells against it, mimicking autoimmune symptoms.
Meta-analysis of prolonged COVID-19 looking at age, gender, comorbidities, ethnicity, and severity of acute disease confirms that female gender, increasing age, and minority ethnicity and Long-COVID risk are associated [41]. However, some of these results have been inconsistent [58]. Studies have shown that Long-COVID syndrome effects with previously hospitalized and non-hospitalized patients are similar. Perhaps, the best known study from the Office for National Statistics (ONS) study including controlled participants reports that the most common symptoms persisting for 12 or more weeks include fatigue (8.3%), headaches (7.2%), cough (7%), and myalgias (5.6%) [59].
A lack of case-controlled studies presents a direct attribution of symptoms that are solely related to COVID-19; however, larger prospective studies with match-controlled groups are needed. As an example, previously stated, the age association may actually be a function of increased number of comorbidities, so is a lack of sufficient access to healthcare. Similarly, many of the studies and statistics on Long-COVID syndrome are based on Long-COVID cohorts produced in Western Europe on patients recently discharged from the hospital. As a result, there was not enough information on Long-COVID patients in low- to middle-income countries and people who were not hospitalized. Also, there are no large studies that focus on children, specifically. This is despite the fact that young children are affected and may be affected by Long-COVID [41].
The Long-COVID syndrome is a complex condition with prolonged heterogenous symptoms that are related to multiple organ involvement. Long-COVID syndrome involve hyperinflammatory and hypercoagulable states that affect all organ systems. It reflects a maladaptation of the angiotensin-converting enzyme 2 (ACE 2) pathway [60, 61]. ACE receptors are present in virtually every organ system. Therefore, it is understandable that many organ systems are susceptible to involvement from direct viral infection and inflammation. As previously mentioned, depending on the duration of symptoms, Long-COVID syndrome may be divided into two stages post-acute COVID or symptoms extending beyond 3 weeks but less than 12 weeks and chronic or Long-COVID where symptoms extend beyond the 12 weeks [62].
Multiple organ sequelae and abnormalities of COVID-19 that may go beyond the acute phase of infection are increasingly being noted as data are being collected. Assessment of key clinical, serological, and imaging features is important to understand the natural history of this disease as it progresses beyond the acute viral infection phase. Workup consisting of pulmonary function tests, chest X-rays, 6-min walk tests, pulmonary embolism workups (when needed), echocardiograms (even serially), and (at times) high-resolution CT scans (to assess for fibrosis) should also be considered. Hematological assessment may lead to extending thromboprophylaxis against clots and high-risk survivors. A neuropsychiatric screening for anxiety, post-traumatic stress, sleep disorders, depression, cognitive impairment, memory abnormalities, and other factors associated with brain fog is important. A neuropsychiatric screening should include a full autonomic dysfunction test, especially in patients with orthostatic intolerance symptoms and chronic fatigue syndromes. Amnesia, sore throat, hoarseness, runny nose, hair loss, hearing loss, and tremors have been reported at low incidences in Long-COVID syndrome [63]. If there are renal function abnormalities, Nephrology follow-up and creatinine clearance determination with urinalysis evaluation may be needed. These may be performed in-person or on virtual clinical visits. Of course in the following discussions, the neuro-cardiovascular effects are compounded by any lingering pulmonary dysfunction causing poor oxygen exchange.
Cardiac Involvement
Common cardiac problems may occur with labile heart rate and blood pressure responses to activity. Myocarditis and pericarditis may occur chronically [64,65,66]. In the acute stages, myocardial infarction, cardiac failure, life-threatening arrhythmias, and sudden cardiac death have been described. The incidents of arrhythmias in Long-COVID syndrome are unknown, but many individuals have palpitations, and studies using ambulatory monitoring need to be further conducted. Sequelae from acute COVID may occur, such as coronary artery aneurysm, aortic aneurysm, atherosclerosis, and venous and arterial thrombotic disease including life-threatening pulmonary embolism [67]. These structural abnormalities may manifest itself in Long-COVID syndrome long after recovery of acute illness and predispose to arrhythmias, breathlessness, and acute coronary events, such as heart attacks and chest pain syndromes [41].
Myocardial injury is the most common abnormality detected with acute COVID infection [68]. It is usually detected even when patients with no cardiac symptoms demonstrate elevated cardiac troponin levels, which may be evident in a high percentage of patients with COVID-19 [69]. Further research is ongoing as to whether this myocardial injury pattern, even when subclinical, may lead to increased arrhythmias and heart failure in the long-term. Echocardiographic studies have shown abnormalities with COVID-19, including right ventricular dysfunction 26.3%, left ventricular dysfunction 18.4%, diastolic dysfunction 13.2%, and pericardial perfusion 7.2%. To what extent this is reversible in patients who go on to Long-COVID syndrome is not known [70]. In addition, sleep abnormalities and difficulties that reduce quality of life have been noted in Long-COVID syndrome patients. These may also adversely affect cardiac function, provoke arrhythmias, elevate blood pressure, and exacerbate or cause hypertensive states [41].
Chest pain and palpitations are status post-acute phase of COVID-19. Palpitations were reported in 9% and chest pain in 5% of patients 6 months after follow-up [71]. To track heart inflammation, one of the most effective and sensitive tests is cardiac magnetic resonance imaging (MRI). Inflammation rates may be as high as 60% more than 2 months after a diagnosis of COVID, although this is a very difficult test to obtain in many centers that do not have it readily available [65]. Long-COVID syndrome patients may present with chest pain in 17% of patients, palpitations in 20% of patients, and dyspnea on exertion 30% of patients [70].
The question of myocarditis is always raised especially in children [34], but adults are also known to have myocarditis. One small study showed that in healthy college athletes with mild COVID-19 symptoms, 15% had evidence of MRI findings consistent with myocarditis on a screening study [72]. More importantly, many of the chest pains and palpitations, which appear to be cardiology in etiology, are actually due to autonomic dysfunction, including the postural orthostatic tachycardia syndrome (POTS) [41]. Therefore, the importance of not only doing cardiac imaging, ambulatory monitoring, stress testing, 6-min walk test, echocardiography, and other noninvasive cardiac workup, but also autonomic testing, such as cardiorespiratory monitoring, HRV interval testing, beat-to-beat blood pressure with tilt testing, and sudomotor testing may be useful in diagnosing autonomic nervous dysfunction. Arrhythmias are noted with Long-COVID, but attention to the use of anti-arrhythmic drugs, amiodarone, for example, must be used carefully in patients who have fibrotic pulmonary changes after COVID-19 [73].
Neurocardiologic Involvement
Neurocardiology focuses on the interaction of the cardiovascular system and the P&S nervous systems. We have discussed how COVID-19 may affect the nervous system in previously unexpected ways [74]. Mitochondrial damage, especially in the P&S nervous systems, in turn affects the control and coordination of all systems throughout the whole body and may explain symptoms of Long-COVID syndrome [25••]. The association of anxiety, POTS, and chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME), with Long-COVID, is interesting because all are also characterized by P&S dysfunction [41]. In general, autonomic dysfunction is the root of neurocardiologic abnormalities. A primary result of autonomic dysfunction in neurocardiologic abnormalities is poor tissue perfusion, especially of the brain and structures above the heart, when upright. Clinical manifestations of these abnormalities may lead to orthostatic dysfunction (POTS or orthostatic hypotension) or syncope. Abnormalities in the P&S nervous systems are often associated with symptoms including fatigue, lightheadedness, “brain-fog,” memory, cognitive, or sleep difficulties; symptoms of depression or anxiety; exercise intolerance; and frequent headache or migraine [57].
Prolonged brain-fog may occur for several months after acute COVID infection [75]. Three months after follow-up MRI, abnormalities may be demonstrated [76]. Brain-fog associated with Long-COVID may involve mechanisms of cardiac deconditioning [77,78,79], post-traumatic stress [80,81,82], or dysautonomia. Long-term cognitive defects may be seen occurring in up to 20–40% of patients [83,84,85]. The association between Long-COVID syndrome and brain-fog may be the result of the autonomic dysfunction, specifically sympathetic withdrawal (an alpha-adrenergic dysfunction) leading to orthostatic dysfunction [86], or parasympathetic excess leading to vasovagal syncope [56] and the associated decreased cerebral perfusion.
Neuropsychiatric sequelae are often common and reported with many post-viral symptoms, such as chronic tiredness, myalgias, depressive symptoms, and non-restorative sleep [87]. Psychiatric manifestations are also common in COVID-19 survivors of more than 1 month. In an Italian study, approximately 15% have at least some evidence of depression and post-traumatic stress, anxiety, insomnia, and obsessive compulsive behavior [88]. Some studies have shown up to 30–40% of patients with COVID-19 have depression and anxiety for prolonged periods of time [89]. Migraine headaches, often refractory to treatment, and late-onset headaches have been presumed to be due to high cytokine levels [90]. In a follow-up study of 100 patients, 38% had ongoing headaches after 6 weeks [91]. Tension headache is common due to “coat-hanger” pain (muscle pain between the shoulders and up the neck) that often leads to headache. “Coat-hanger” pain is a function of poor perfusion of the muscles involved. Loss of taste and smell (potentially as a result of poor perfusion and oxidative stress of the corresponding cranial nerves) may also persist for up to 6 months and longer on follow-up of patients. Brain-fog, cognitive impairment, concentration, memory difficulties, receptive language, and executive function abnormalities may also persist over a long period of time. Neuropsychiatric manifestations of COVID-19 have been documented in a British study including stroke and altered mental status. Multiple psychiatric symptoms emanating from encephalopathy and encephalitis and primary psychiatric diagnoses were noted in young adults [92]. This may be related to autonomic dysfunction and other factors [93, 94].
After ischemic or hemorrhagic strokes, hypoxic and endemic damage may lead to reversible encephalopathy syndrome and acute disseminated myelitis. These and other neurological defects may require extensive rehabilitation and their sequelae may linger. Also, acute critical illness, myopathy, and neuropathies resulting during acute COVID-19 may leave residual symptoms weeks to months later [95].
Autonomic dysfunction has been noted to be significant. Patients with orthostatic tachycardia and inappropriate sinus tachycardia may benefit from heart rate management including beta-blockers [96] and other autonomic therapies (personal observations). Prolonged effects on patients who have had prone ventilation and COVID-19 may have focal and multifocal peripheral nerve damage [97]. Symptoms consistent with orthostatic hypotension syndrome and painful small fiber neuropathy were reported in as short as 3 weeks and as long as 3 months [98,99,100,101]. Muscle wasting and fragility are often seen prolonged. This is because COVID-19 when it is severe may cause catabolic muscle weakness and feeding difficulties [102]. The most frequent neurological long-term symptoms in patients were myalgias, arthralgias, sleeping troubles, and headaches [42].
Autonomic dysfunction findings resulting from analysis of COMPASS 31 Questionnaire demonstrate significant symptomatology [103]. Based on autonomic symptoms by COMPASS 31, two clusters were obtained with differential characteristics. The first cluster was discussed above. Cluster 2 exhibited high scores of COMPASS 31. This accounted for 31% of all patients included. Median COMPASS 31 score of 22 suggests that one third of patients with Long-COVID may yield higher scores when compared with the general population [104]. Patients with higher scores exhibited more clinical manifestations and depression. These clusters have therapeutic implications, and clinicians should be aware of particular manifestations during the follow-up, and all psychosocial intervention may reduce the burden of Long-COVID syndrome [105].
Orthostatic intolerance has been described from a series of patients that had either resting or postural hypotension or tachycardia [98]. Orthostatic intolerance symptoms presented from orthostatic hypotension, vasovagal syncope, and POTS that all occur and last for a prolonged period of time after COVID-19 viral infection. The symptoms are often associated with palpitations, breathlessness, and chest pain, which are common symptoms seen in Long-COVID patients. It is postulated that high catecholamine levels may lead to paradoxical vasal dilatation and increased activation of the vagus nerve that may result even in syncope and also sympathetic withdrawal [106, 107].
Hypovolemia may also exacerbate or worsen these symptoms. The relationship between COVID-19 infection and how it affects the autonomic nervous system is not completely understood, but it is believed to be due to inflammatory cytokine release and a cytokine storm, which results from the initial sympathetic activation produced by a pro-inflammatory cytokine release [108]. However, vagal stimulation (i.e., in coordination with the immune response to COVID-19) may also result in anti-inflammatory responses [109]. The COVID-19 relationship to autonomic dysfunction may be by the viral infection itself. Immuno-mediated neurological syndromes have been described [110]. It is well-known that some forms of orthostatic hypotension and POTS are associated with autoantibodies perhaps caused by other viruses in the past, and this may also be the case with COVID; for example, antibodies against alpha and beta and muscarinic receptors.
Treatment of these P&S nervous system disorders involves exercise with both aerobic and resistant elements in a graded, or “low-and-slow,” fashion that oftentimes begin with recumbent exercises [111]. Fluid and electrolyte repletion is required. Avoiding exacerbating factors, such as prolonged sitting and warm environments, is recommended. Some counter-maneuvers and isometric exercises, compression garments especially up to the waist, or abdominal binders are recommended. Pharmacological treatment that may involve different regimens, such as volume expanders (e.g., fludrocortisone or desmopressin), may be used along with oral vasoactivation (e.g., midodrine, Mestinon, or droxidopa) [112]. If there are prominent hyperadrenergic symptoms, propranolol, clonidine, methyldopa, or other beta-blockers may be considered, especially for a POTS response.
Again, the questionnaire that addresses autonomic symptoms, such as the composite autonomic symptom scale-31 (COMPASS 31), may be used to validate dysautonomia symptoms in post-COVID patients. In a study of 180 participants (70% males) [113], orthostatic hypotension was found in 13.8% of the subjects. The mean COMPASS 31 score was 17.6 with the most affected domains being orthostatic intolerance, sudomotor, gastrointestinal, and pupillomotor dysfunction. Higher COMPASS 31 scores were found in those with neurological symptoms due to more severe orthostatic intolerance symptoms. Interestingly, gastrointestinal, urinary, and pupillomotor domains were more represented than non-neurological symptom groups. Therefore, this study concluded that it is important to monitor autonomic nervous system symptoms as a complication of COVID-19 disease that may persist in a post-acute period. Other prominent autonomic dysfunctions in these post-COVID individuals included secretomotor and sweating abnormalities in about half the study population and thermoregulatory alterations.
Another feature of COVID-19 involves the urinary tract and bladder incontinence. Urinary symptoms may occur with inflammation and demyelination of the pudendal nerve. This has been reported in other viral infections [114, 115]. Up to a half of Long-COVID syndrome patients may have urinary dysfunction [113]. It is also suggested that sexual impairment such as erectile dysfunction is possible after COVID infections which may affect both endothelial and autonomic components. However, the psychiatric component due to stress and anxiety cannot be separated [116, 117].
COMPASS 31 scoring highlights gastrointestinal (GI) symptoms after COVID-19. These may occur along with a combination of other symptoms, including abnormal appetite, nausea, and diarrhea. This is because in the GI tract, there is a significant amount of angiotensin coenzyme ACE-2 receptors present [118]. Also, the parasympathetics control the majority of the GI tract and may be responsible for motility dysfunction due to parasympathetic dysfunction [56].
Vision disturbances may also occur in Long-COVID syndromes including sore eyes and light sensitivity [119]. In acute conjunctivitis, an abnormal pupil response has been noted [120]. Light sensitivity is often a common complaint. Nearly 50% of study patients [113] complained of ocular abnormalities. Therefore, multiple organ systems may manifest symptoms in Long-COVID syndrome which may be attributed to the autonomic dysfunction, and this need not necessarily just be orthostatic intolerance symptoms [121]. As noted, GI, ocular, urological, sudomotor, and endocrine organs may be affected.
Dysautonomia, including anosmia, hypogeusia, headaches, and hypoxia, has been shown to be components of the Long-COVID syndrome [122]. The dysautonomia is hypothesized to occur secondary to COVID-19 infection. The demonstrated dysregulation of heart rate variability (HRV) testing is reflected in abnormal nociception level (NOL) index, an index that refers to abnormal parasympathetic-sympathetic balance. The NOL algorithm with a multiparameter, non-linear combination of (1) heart rate, (2) HRV, (3) amplitude of the finger, photoplethysmograph, skin conduction level, and (4) time derivatives were obtained using a noninvasive finger probe [122].
Autonomic dysfunction has been reported in up to 63% of patients presenting with specific symptoms having survived a COVID-19 infection between March 2020 and January 2021. Diagnoses were revealed by recording and evaluating beat-to-beat blood pressure and HRV during head-up tilt [123]. As long as 6 to 8 months after recovery from COVID-19, patients still suffer residual autonomic symptoms, and 60% are unable to return to work, and only 50% have completely recovered [124].
Chronic fatigue syndrome, also known as post-infective syndrome, has been commonly recognized in the Long-COVID syndrome. A study of 20 fatigued and 20 non-fatigued Long-COVID patients with a mean age 44.5 (90% female) was followed up for 166 days. A scale known as a Chalder fatigue scale was used. They underwent a Ewing autonomic function test battery, including deep breathing, active standing, Valsalva, and cold pressure testing, with continuous electrocardiogram, blood pressure monitoring, and cerebral oxygenation. A 24-h ambulatory blood pressure monitoring was conducted. Findings included no differences between the fatigued and non-fatigued patients in autonomic testing. There was an increase in anxiety, strongly associated with chronic fatigue syndrome. This, however, is in contradistinction to other studies which have shown autonomic dysfunction with Long-COVID syndrome and chronic fatigue [125]. Much of this confusion is attributable to the fact that the autonomic measures used do not simultaneously and independently differentiate parasympathetic from sympathetic activity [57].
Post-infectious myalgic encephalomyelitis, which is also synonymous with chronic fatigue syndrome, has been recognized along with sleep disturbances, neurocognitive changes, orthostatic intolerance, and post-exertional malaise [126, 127]. Fatigue at 3 weeks post-symptoms may occur in 13–33% of patients. There are many factors responsible which include sleep disturbances, autonomic dysfunction with sympathetic predominance, endocrine disturbances, abnormalities of the hypothalamic–pituitary–adrenal axis, reactive mood disorders, and depression and anxiety. Findings therefore concluded that chronic fatigue post Long-COVID syndrome is multifactorial. More testing is required to see the association of autonomic disturbances with the emergence of a chronic fatigue syndrome. This has been demonstrated with other viral infections. In fact, in the cohort at our autonomic clinic, we have found significant disturbances in cardiorespiratory and heart rate variability testing in patients with chronic fatigue with Long-COVID syndrome with abnormal autonomic responses, including sympathetic withdraw (associated with orthostatic dysfunction) and vagal excess with postural change (associated with pre-syncope symptoms). Both autonomic dysfunctions are associated with poor cerebral and possibly coronary perfusion. These symptoms present regardless of whether they have drops in blood pressure, postural rise in heart rate, or none of the above changes.
It has been widely reported that autonomic dysfunction and various viral infections including HIV, herpes virus, enterovirus 71, flavivirus, and human T lymphotropic virus may result in loss of heart rate variability, hypersalivation, photophobia, and dyspnea. Epstein–Barr virus may lead to dysautonomia through alterations of cerebral autoregulation by high nitric oxide release with subsequent autonomic nervous system dysfunction. It has been postulated that the coronavirus shares a common feature of neuro-invasion [128]. SARS-CoV-2 may also reach the autonomic nervous system (1) by employing the retrograde axonal transport by the olfactory nerve [129], (2) via ACE 2 receptors and brain stem pathways [130], (3) via systemic blood circulation [131], (4) by immune injury [132], and (5) by neuronal pathways [133]. The virus may also indirectly invade the autonomic nervous system by the enteric nervous system (ENS) and its sympathetic afferent neurons by infecting the GI tract [134, 135]. In a study of 50 patients, it showed that 26% of patients had sweat dysfunction as measured by SUDOSCAN test with motor, sensory, and autonomic dysfunction [136]. Abnormalities of HRV are more common in COVID-19 patients who develop fatigue [137, 138]; Long-COVID is found to be associated with persistent symptoms, such as hypoxia and fatigue due to development of dysautonomia [139]. It is initially believed that COVID-19 causes sympathetic nervous system activation with catecholamine excess of activation in a sympathetic storm which activates the renin angiotensin system. Simultaneously, there is inhibition of the parasympathetic nervous system-mediated anti-inflammatory effect that leads to a decrease in neuro-vagal anti-inflammatory response and enhances the cytokine storm. This all leads to cardiopulmonary complications and COVID-19-induced dysautonomia [135].
The parasympathetic responses reported above are found at rest. There is an abnormal parasympathetic response to stress that may also occur [56], exaggerating the dynamic sympathetic response to stress, thereby amplifying and prolonging the sympathetically mediated inflammatory, histaminergic, pain, and anxiety responses in a post-traumatic-like fashion.
Pathophysiology of Long-COVID
The mechanism behind the causation of Long-COVID syndrome may be multifactorial [26, 28, 140, 141]. Immune response, antibody generations, direct effects of the virus, complications of the critical illness, psychosocial factors, and post-intensive care syndrome, post-traumatic stress, and oxidative stress may be operative mechanisms. Cardiac deconditioning may also be a factor [79]. The mechanism of heart failure involving pro-inflammatory cytokines with interleukin 1 and interleukin 6 tumor necrosis factors may cause prolonged effects [142]. Redox imbalance linking COVID-19 and chronic fatigue syndromes and systemic inflammation and neuroinflammation have also been postulated [143].
Oxidative phosphorylation may be operative in a hyperinflammatory state with altered cardiorespiratory function. It is thought that viral infections cause a shift in mitochondrial energy system contribution from ATP synthesis to innate immune signaling occurring in order to eradicate pathogens, promote inflammation, and eventually restore tissue homeostasis [144]. An increased rate of glycolysis and downregulation of oxidative phosphorylation are seen [145]. Oxidative stress has been implicated in many acquired myocardial disorders and may lead to significant autonomic dysfunction [146•].
Treatment in Long-COVID Syndrome
For the most part, supportive therapy for Long-COVID symptoms is a keystone, and there is treatment for autonomic dysfunction that may be demonstrated objectively in a laboratory. As mentioned earlier, volume expanders and oral-vasoactives, in addition to fluids, electrolytes, compression garments, and various exercise techniques, have been prescribed for orthostatic intolerance symptoms [112, 146•]. Omega-3 fatty acids and dietary supplementation have been investigated [147]. It is believed that omega-3 s may help resolve inflammatory imbalance. L-Arginine has also been proposed as a treatment modality [148]. Included in the L-arginine pathway is the production of nitric oxide. Nitric oxide maintains or improves the health and function of endothelial cells and benefits the immune system, especially in chronic fatigue states [146•]. Various antioxidants [146•], including zinc, have also been used empirically, but there are no controlled studies to confirm their utility. We have found alpha-lipoic acid, used as an antioxidant to relieve autonomic neuropathy in populations of patients diagnosed with type 2 diabetes mellitus [149,150,151,152], to be effective in Long-COVID patients. Electrical neuro-prostheses stimulating either the parasympathetic (vagus) or sympathetic nervous system have been known to help relieve symptoms of autonomic dysfunction [153, 154]. All of these therapies also effect proper autonomic function to help relieve Long-COVID symptoms [25••]. Vaccination has been suggested as possibly a factor that may ease symptoms of Long-COVID. In one large survey, 57% of responders reported an overall improvement in their symptoms following vaccination, and around 19% reported an overall deterioration [155]. Mental health conditions may be treated with various psychological aides, such as cognitive behavioral therapy as well as antidepressants, including tricyclics. Treatment of liver function abnormalities, irritable bowel syndrome, dyspepsia, and other GI symptoms is very challenging. Renal dysfunction should be followed serially, nephrotoxins avoided, and proper hydration maintained. However, from a large study of fully vaccinated people, 2.6% were found to contract the virus (labeled as “breakthrough” patients) [156]. Among 1497 fully vaccinated healthcare workers for whom RT-PCR data were available, 39 SARS-CoV-2 breakthrough infections were documented. Neutralizing antibody titers in case patients during the peri-infection period was lower than those in matched uninfected controls (case-to-control ratio, 0.361; 95% confidence interval, 0.165 to 0.787). Higher peri-infection neutralizing antibody titers were associated with lower infectivity (higher Ct values). Most breakthrough cases were mild or asymptomatic, although 19% had persistent symptoms (> 6 weeks). The B.1.1.7 (alpha) variant was found in 85% of samples tested. A total of 74% of case patients had a high viral load (Ct value, < 30) at some point during their infection; however, of these patients, only 17 (59%) had a positive result on concurrent Ag-RDT. No secondary infections were documented. Among fully vaccinated healthcare workers, the occurrence of breakthrough infections with SARS-CoV-2 was correlated with neutralizing antibody titers during the peri-infection period. Most breakthrough infections were mild or asymptomatic, although persistent symptoms did occur.
Conclusion
Long-COVID symptoms [157] may be explained by a pro-inflammatory state with oxidative stress and P&S dysfunction [46]. Cardiopulmonary testing for unexplained dyspnea post-COVID-19 was reported [158]. Patients with symptoms consistent with chronic fatigue had an abnormal pattern of oxygen uptake on cardiopulmonary testing consistent with what is seen with chronic fatigue syndrome. Circulatory impairment, abnormal ventilatory pattern, and chronic fatigue syndrome may be common in patients with post-acute sequelae of post-concussive syndrome, and this accounts for the mechanism of dyspnea in many patients who do not have pulmonary disease from Long-COVID or myocardial dysfunction. The symptoms of Long-COVID syndrome may all be associated with autonomic dysfunction as measured with cardiorespiratory testing [25••] and relieved with appropriate parasympathetic or sympathetic therapies based on the cardiorespiratory test [146•].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–46. https://doi.org/10.1016/j.jacc.2020.06.007.
Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–48.
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–10. https://doi.org/10.1001/jamacardio.2020.0950.
Lu JQ, Lu JY, Wang W, Liu Y, Buczek A, Fleysher R, et al. Clinical predictors of acute cardiac injury and normalization of troponin after hospital discharge from COVID-19: predictors of acute cardiac injury recovery in COVID-19. EBioMedicine. 2022;76:103821. https://doi.org/10.1016/j.ebiom.2022.103821.
Mikkelsen ME, Abramoff B. COVID-19: Evaluation and management of adults following acute viral illness - UpToDate. Available at: https://www.uptodate.com/contents/covid-19-evaluation-and-management-of-adults-following-acute-viral-illness. Accessed 1 Jul 2022.
Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 2020; 6.
Greenhalgh T, Knight M, A’Court C, et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370:m3026.
COVID-19 rapid guideline: managing the long-term effects of COVID-19, National Institute for Health and Care Excellence (UK). 2022.
Radtke T, Ulyte A, Puhan MA, Kriemler S. Long-term symptoms after SARS-CoV-2 infection in children and adolescents. JAMA 2021.
Vanichkachorn G, Newcomb R, Cowl CT, et al. Post-COVID-19 syndrome (long haul syndrome): description of a multidisciplinary clinic at Mayo Clinic and characteristics of the initial patient cohort. Mayo Clin Proc. 2021;96:1782.
Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022. https://doi.org/10.1093/infdis/jiac136.
Tada Y, Suzuki J. Oxidative stress and myocarditis. Curr Pharm Des. 2016;22(4):450–71. https://doi.org/10.2174/1381612822666151222160559. PMID: 26696256.
Lüscher TF. Ageing, inflammation, and oxidative stress: final common pathways of cardiovascular disease. Eur Heart J. 2015;36(48):3381–3. https://doi.org/10.1093/eurheartj/ehv679. PMID: 26690751.
Mito S, Thandavarayan RA, Ma M, Lakshmanan A, Suzuki K, Kodama M, et al. Inhibition of cardiac oxidative and endoplasmic reticulum stress-mediated apoptosis by curcumin treatment contributes to protection against acute myocarditis. Free Radic Res. 2011;45(10):1223–31. https://doi.org/10.3109/10715762.2011.607252. Epub 2011 Aug 18 PMID: 21781008.
Iddir M, Brito A, Dingeo G, Fernandez Del Campo SS, Samouda H, La Frano MR, et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12(6):1562. https://doi.org/10.3390/nu12061562. PMID:32471251;PMCID:PMC7352291.
Delgado-Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS-CoV) infection. Arch Med Res. 2020;51(5):384–7. https://doi.org/10.1016/j.arcmed.2020.04.019. Epub 2020 Apr 30. PMID: 32402576; PMCID: PMC7190501.
Lee C. Therapeutic modulation of virus-induced oxidative stress via the Nrf2-dependent antioxidative pathway. Oxid Med Cell Longev. 2018;31(2018):6208067. https://doi.org/10.1155/2018/6208067. PMID:30515256;PMCID:PMC6234444.
Suhail S, Zajac J, Fossum C, Lowater H, McCracken C, Severson N, et al. Role of oxidative stress on SARS-CoV (SARS) and SARS-CoV-2 (COVID-19) infection: a review. Protein J. 2020;39(6):644–56. https://doi.org/10.1007/s10930-020-09935-8. Epub 2020 Oct 26. PMID: 33106987; PMCID: PMC7587547.
Beltrán-García J, Osca-Verdegal R, Pallardó FV, Ferreres J, Rodríguez M, Mulet S, et al. Oxidative stress and inflammation in COVID-19-associated sepsis: the potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants (Basel). 2020;9(10):936. https://doi.org/10.3390/antiox9100936. PMID:33003552;PMCID:PMC7599810.
Saleh J, Peyssonnaux C, Singh KK, Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion. 2020;54:1–7. https://doi.org/10.1016/j.mito.2020.06.008. Epub 2020 Jun 20. PMID: 32574708; PMCID: PMC7837003.
•• Rasa S, Nora-Krukle Z, Henning N, Eliassen E, Shikova E, Harrer T, et al. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. 2018;16(1):268. https://doi.org/10.1186/s12967-018-1644-y. PMID: 30285773; PMCID: PMC6167797. This manuscript provides the basis for the central figure to our review, demonstrating the association between viruses, including COVID-19, and other traumas (both mental and physical), and oxidative stress, which in turn is associated with parasympathetic and sympathetic dysfunctions known as dysautonomias that are associated with Long-COVID syndrome.
da Cunha NV, Lopes FN, Panis C, Cecchini R, Pinge-Filho P, Martins-Pinge MC. iNOS inhibition improves autonomic dysfunction and oxidative status in hypertensive obese rats. Clin Exp Hypertens. 2017;39(1):50–7. https://doi.org/10.1080/10641963.2016.1210628. Epub 2017 Jan 5 PMID: 28055264.
Hoeldtke RD, Bryner KD, VanDyke K. Oxidative stress and autonomic nerve function in early type 1 diabetes. Clin Auton Res. 2011;21(1):19–28. https://doi.org/10.1007/s10286-010-0084-4. Epub 2010 Sep 25 PMID: 20872157.
Ziegler D, Buchholz S, Sohr C, Nourooz-Zadeh J, Roden M. Oxidative stress predicts progression of peripheral and cardiac autonomic nerve dysfunction over 6 years in diabetic patients. Acta Diabetol. 2015;52(1):65–72. https://doi.org/10.1007/s00592-014-0601-3. Epub 2014 Jun 5 PMID: 24898524.
•• Colombo J, Weintraub MI, Munoz R, Verma A, Ahmed G, Kaczmarski K, et al. Long-COVID and the autonomic nervous system: the journey from dysautonomia to therapeutic neuro-modulation, analysis of 152 patient retrospectives. NeuroSci. 2022;3(2):300–10. https://doi.org/10.3390/neurosci3020021. This manuscript presents the symptoms and therapies of a population of autonomic dysfunction patients who subsequently became COVID-19 survivors. These patients were tracked before and after acute COVID-19 infection. The monitoring, assessment, diagnoses, and therapies presented in this previously published study form the kernel to the subject of this current review article on Long-COVID syndrome.
Dixit NM, Churchill A, Nsair A, Hsu JJ. Post-acute COVID-19 syndrome and the cardiovascular system: what is known? Am Heart J Plus. 2021;5:100025. https://doi.org/10.1016/j.ahjo.2021.100025. Epub 2021 Jun 24. PMID: 34192289; PMCID: PMC8223036.
Wu X, Deng KQ, Li C, Yang Z, Hu H, Cai H, et al. Cardiac involvement in recovered patients from COVID-19: a preliminary 6-month follow-up study. Front Cardiovasc Med. 2021;13(8):654405. https://doi.org/10.3389/fcvm.2021.654405. PMID:34055936;PMCID:PMC8155269.
Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43(11):1157–72. https://doi.org/10.1093/eurheartj/ehac031. PMID:35176758;PMCID:PMC8903393.
Nascimento BR, Sable C. Cardiac involvement in COVID-19: cause or consequence of severe manifestations? Heart. 2022;108(1):7–8. https://doi.org/10.1136/heartjnl-2021-320246. Epub 2021 Oct 16 PMID: 34656975.
Abbasi J. The COVID heart-one year after SARS-CoV-2 infection, patients have an array of increased cardiovascular risks. JAMA. 2022;327(12):1113–4. https://doi.org/10.1001/jama.2022.2411. PMID: 35234824.
Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–90. https://doi.org/10.1038/s41591-022-01689-3. Epub 2022 Feb 7 PMID: 35132265.
Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV III, et al. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res. 2021;128(8):1214–36. https://doi.org/10.1161/CIRCRESAHA.121.317997. Epub 2021 Apr 15. PMID: 33856918; PMCID: PMC8048382.
Gasecka A, Pruc M, Kukula K, Gilis-Malinowska N, Filipiak KJ, Jaguszewski MJ, et al. Post-COVID-19 heart syndrome. Cardiol J. 2021;28(2):353–4. https://doi.org/10.5603/CJ.a2021.0028. Epub 2021 Mar 1. PMID: 33645626; PMCID: PMC8078939.
Aeschlimann FA, Misra N, Hussein T, Panaioli E, Soslow JH, Crum K, et al. Myocardial involvement in children with post-COVID multisystem inflammatory syndrome: a cardiovascular magnetic resonance based multicenter international study-the CARDOVID registry. J Cardiovasc Magn Reson. 2021;23(1):140. https://doi.org/10.1186/s12968-021-00841-1. PMID:34969397;PMCID:PMC8717054.
Gale J. Heart damage plagues COVID survivors a year after infection, study shows. 12:10 AM EST, 2021. https://www.bloombergquint.com/coronavirus-outbreak/heart-damage-racks-covid-survivors-a-year-after-infection-study. Accessed 15 Feb 2022.
Komiyama M, Hasegawa K, Matsumori A. Dilated cardiomyopathy risk in patients with coronavirus disease 2019: how to identify and characterise it early? Eur Cardiol. 2020;27(15):e49. https://doi.org/10.15420/ecr.2020.17. PMID:32536978;PMCID:PMC7277785.
Revzin MV, Raza S, Srivastava NC, Warshawsky R, D’Agostino C, Malhotra A, et al. Multisystem imaging manifestations of COVID-19, part 2: from cardiac complications to pediatric manifestations. Radiographics. 2020;40(7):1866–92. https://doi.org/10.1148/rg.2020200195. PMID: 33136488; PMCID: PMC7646410.
Patel RD, Chen K, Kyriakakos C, Pellerito JS. Multisystem imaging manifestations of COVID-19, part 2: from cardiac complications to pediatric manifestations. Radiographics. 2020;40(7):1866–92. https://doi.org/10.1148/rg.2020200195. PMID: 33136488; PMCID: PMC7646410.
Centers for disease control and prevention. Post-COVID conditions: information for healthcare providers. 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/post-covid-conditions.html. Accessed 1 Jul 2022.
World Health Organization. ICD-10 : international statistical classification of diseases and related health problems: tenth revision, 2nd ed. World Health Organization. 2004. https://apps.who.int/iris/handle/10665/42980. Accessed 15 Feb 2022.
DePace NL, Colombo J. Long-COVID syndrome: a multi-organ disorder. Cardio Open. 2022;7(1):213–24. https://doi.org/10.33140/COA.07.01.07.
Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med (Lausanne). 2021;8:653516. https://doi.org/10.3389/fmed.2021.653516. PMID: 34017846; PMCID: PMC8129035.
World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1.
Afrin LB, Self S, Menk J, Lazarchick J. Characterization of mast cell activation syndrome. Am J Med Sci. 2017;353(3):207–15. https://doi.org/10.1016/j.amjms.2016.12.013.
Afrin LB, Weinstock LB, Molderings GJ. Covid-19 hyperinflammation and post-COVID-19 illness may be rooted in mast cell activation syndrome. Int J Infect Dis. 2020;100:327–32. https://doi.org/10.1016/j.ijid.2020.09.016.
Bisaccia G, Ricci F, Recce V, Serio A, Iannetti G, Chahal AA, et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J Cardiovasc Dev Dis. 2021;8(11):156. https://doi.org/10.3390/jcdd8110156. PMID:34821709;PMCID:PMC8621226.
Perego E. The Long-COVID, COVID-19 is starting to be addressed on major newsapapers in Italy too: ~20% of tested patients remain COVID+ for at least 40 days. Professor from Tor Vergata University of Rome notes: there is a lot we don’t know about this virus. 20 May 2020. https://twitter.com/elisaperego78/status/1263172084055838721. Accessed 15 Feb 2022.
NBC Nightly News. COVID-19 ‘longhaulers’ report nearly 100 symptoms for more than 100 days. 20 July 2020. https://www.nbcnews.com/nightly-news-netcast/video/nightly-news-full-broadcast-july-31st-89377349826. Accessed 15 Feb 2022.
Yong E. Even health-care workers with Long-COVID are being dismissed. 24 November 2021. https://www.theatlantic.com/health/archive/2021/11/health-care-workers-long-covid-are-being-dismissed/620801/. Accessed 15 Feb 2022.
Garg P, Arora U, Kumar A, Wig N. The, “post-COVID” syndrome: how deep is the damage? J Med Virol. 2021;93(2):673–4. https://doi.org/10.1002/jmv.26465. Epub 2020 Sep 29. PMID: 32852801; PMCID: PMC7461449.
Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr. 2021;15(3):869–75. https://doi.org/10.1016/j.dsx.2021.04.007. Epub 2021 Apr 20. PMID: 33892403; PMCID: PMC8056514.
Carfì A, Bernabei R, Landi FG. Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–5. https://doi.org/10.1001/jama.2020.12603.
Nabavi N. Long COVID: how to define it and how to manage it. BMJ. 2020;7(370):m3489. https://doi.org/10.1136/bmj.m3489. PMID: 32895219.
Cirulli ET, Barrett KMS, Riffle S, Bolze A, Neveux I, Dabe S, et al. Long-term COVID-19 symptoms in a large unselected population. medRxiv. 2020. https://doi.org/10.1101/2020.10.07.20208702.
Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur. 2021;6:100122. https://doi.org/10.1016/j.lanepe.2021.100122. PMID: 34027514; PMCID: PMC8129613.
Tobias H, Vinitsky A, Bulgarelli RJ, Ghosh-Dastidar S, Colombo J. Autonomic nervous system monitoring of patients with excess parasympathetic responses to sympathetic challenges – clinical observations. US Neurology. 2010;5(2):62–6.
Colombo J, Arora RR, DePace NL, Vinik AI. Clinical autonomic dysfunction: measurement, indications, therapies, and outcomes. Springer Science + Business Media, New York, NY, 2014.
Michelen M, Manoharan L, Elkheir N, Cheng V, Dagens A, Hastie C, et al. Characterising long COVID: a living systematic review. BMJ Glob Health. 2021;6(9):e005427. https://doi.org/10.1136/bmjgh-2021-005427. PMID:34580069;PMCID:PMC8478580.
Ayoubkhani D and Pawelek P. Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK: 1 July 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/1july2021. Accessed 15 Feb 2022.
Monteil V, Kwon H, Prado P, Hagelkrüys A, Wimmer RA, Stahl M, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913.e7. https://doi.org/10.1016/j.cell.2020.04.004.
Qaradakhi T, Gadanec LK, McSweeney KR, Tacey A, Apostolopoulos V, Levinger I, et al. The potential actions of angiotensin-converting enzyme II (ACE2) activator diminazene aceturate (DIZE) in various diseases. Clin Exp Pharmacol Physiol. 2020;47:751–8. https://doi.org/10.1111/1440-1681.13251.
Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute COVID-19 in primary care. BMJ. 2020;11(370):m3026. https://doi.org/10.1136/bmj.m3026. PMID: 32784198.
Aiyegbusi OL, Hughes SE, Turner G, Rivera SC, McMullan C, Chandan JS, et al. Symptoms, complications and management of long COVID: a review. J R Soc Med. 2021;114(9):428–42. https://doi.org/10.1177/01410768211032850. Epub 2021 Jul 15. PMID: 34265229; PMCID: PMC8450986.
Knight DS, Kotecha T, Razvi Y. COVID-19: myocardial injury in survivors. Circulation. 2020;142(11):1120–2. https://doi.org/10.1161/circulationaha.120.049252.Sep15.
Puntmann VO, Carerj ML, Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–73. https://doi.org/10.1001/jamacardio.2020.3557.
Kotecha T, Knight DS, Razvi Y. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021. https://doi.org/10.1093/eurheartj/ehab075.
Becker RC. Toward understanding the 2019 Coronavirus and its impact on the heart. J Thromb Thrombolysis. 2020;50(1):33–42. https://doi.org/10.1007/s11239-020-02107-6. PMID:32297133;PMCID:PMC7156795.
Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, Nasr A, et al. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(3):314–25. https://doi.org/10.1016/j.jacc.2020.11.031.
Sandoval Y, Januzzi JL Jr, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76(10):1244–58. https://doi.org/10.1016/j.jacc.2020.06.068. Epub 2020 Jul 8. PMID: 32652195; PMCID: PMC7833921.
Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M, et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol. 2020;76(18):2043–55. https://doi.org/10.1016/j.jacc.2020.08.069. PMID:33121710;PMCID:PMC7588179.
Zeng JH, Wu WB, Qu JX, Wang Y, Dong CF, Luo YF, et al. Cardiac manifestations of COVID-19 in Shenzhen, China. Infection. 2020;48:861–70. https://doi.org/10.1007/s15010-020-01473-w.
Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–8. https://doi.org/10.1001/jamacardio.2020.4916. Erratum.In:JAMACardiol.2021Jan1;6(1):123.PMID:32915194;PMCID:PMC7489396.
Kociol RD, Cooper LT, Fang JC, Moslehi JJ, Pang PS, Sabe MA, et al. Recognition and initial management of fulminant myocarditis: a scientific statement from the American Heart Association. Circulation. 2020;141(6):e69–92. https://doi.org/10.1161/CIR.0000000000000745. Epub 2020 Jan 6. PMID: 31902242.
Abboud H, Abboud FZ, Kharbouch H, Arkha Y, El Abbadi N, El Ouahabi A. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53. https://doi.org/10.1016/j.wneu.2020.05.193. Epub 2020 May 28. PMID: 32474093; PMCID: PMC7255736.
Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–27. https://doi.org/10.1001/jamaneurol.2020.2065. PMID:32469387;PMCID:PMC7484225.
Lu Y, Li X, Geng D, Mei N, Wu PY, Huang CC, et al. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. https://doi.org/10.1016/j.eclinm.2020.100484. Epub 2020 Aug 3. PMID: 32838240; PMCID: PMC7396952.
Stremel RW, Convertino VA, Bernauer EM, Greenleaf JE. Cardiorespiratory deconditioning with static and dynamic leg exercise during bed rest. J Appl Physiol. 1976;41(6):905–9. https://doi.org/10.1152/jappl.1976.41.6.905.
Joyner MJ, Masuki S. POTS versus deconditioning: the same or different? Clin Auton Res. 2008;18(6):300–7. https://doi.org/10.1007/s10286-008-0487-7.
Lechien JR, Chiesa-Estomba CM, Place S. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288(3):335–44. https://doi.org/10.1111/joim.13089.
Halpin SJ, McIvor C, Whyatt G, et al. Postdischarge symptoms and rehabilitation needs in survivors of COVID-19 infection: a cross-sectional evaluation. J Med Virol. 2020.
Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry. 2020.
Writing Committee for the COMEBAC Study Group, Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA. 2021; PMID 33729425.
Novac E. Neurologicals. 2021;21:100–276.
Miglis MG, Goodman BP, Chémali KR, Stiles L, et al. Re: ‘Post-COVID-19 chronic symptoms’ by Davido. Clin Microbiol Infect. 2021;27(3):494. https://doi.org/10.1016/j.cmi.2020.08.028.
Sakusic A, Rabinstein AA. Cognitive outcomes after critical illness. Curr Opin Crit Care. 2018;24(5):410–4. https://doi.org/10.1097/MCC.0000000000000527. PMID: 30036191.
Arora RR, Bulgarelli RJ, Ghosh-Dastidar S, Colombo J. Autonomic mechanisms and therapeutic implications of postural diabetic cardiovascular abnormalities. J Diabetes Sci Technol. 2008;2(4):568–71.
Fauci A. International AIDS Conference: YouTube 2020 and Nordvigas. Potential neurological manifestations of COVID-19. Neurology Clin Pract. 18 March 2020.
Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. https://doi.org/10.1016/j.bbi.2020.07.037. Epub 2020 Jul 30. PMID: 32738287; PMCID: PMC7390748.
Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–15. https://doi.org/10.1038/s41591-021-01283-z. Epub 2021 Mar 22 PMID: 33753937.
Belvis R. Headaches during COVID-19: my clinical case and review of the literature. Headache. 2020;60(7):1422–6. https://doi.org/10.1111/head.13841. PMID: 32413158; PMCID: PMC7273035.
Pozo-Rosich P. Virtual annual scientific meetings - Advances in Headache science. 17 July 2020.
Varatharaj A, Thomas N, Ellul MA, Davies NWS, Pollak TA, Tenorio EL, et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7(10):875–82. https://doi.org/10.1016/S2215-0366(20)30287-X. Epub 2020 Jun 25. Erratum in: Lancet Psychiatry. 2020 Jul 14;: PMID: 32593341; PMCID: PMC7316461.
Henek A. Alzheimer’s research therapeutics. 2020;1269.
Kaseda ET, Levine AJ. Post-traumatic stress disorder: a differential diagnostic consideration for COVID-19 survivors. Clin Neuropsychol. 2020;34(7–8):1498–514. https://doi.org/10.1080/13854046.2020.1811894. Epub 2020 Aug 26. PMID: 32847484.
Tankisi H, Tankisi A, Harbo T, Markvardsen LK, Andersen H, Pedersen TH. Critical illness myopathy as a consequence of COVID-19 infection. Clin Neurophysiol. 2020;131(8):1931–2. https://doi.org/10.1016/j.clinph.2020.06.003. Epub 2020 Jun 12. PMID: 32619798; PMCID: PMC7834604.
Raj SR, Black BK, Biaggioni I, Paranjape SY, Ramirez M, Dupont WD, et al. Propranolol decreases tachycardia and improves symptoms in the postural tachycardia syndrome: less is more. Circulation. 2009;120(9):725–34. https://doi.org/10.1161/CIRCULATIONAHA.108.846501. Epub 2009 Aug 17. PMID: 19687359; PMCID: PMC2758650.
Malik G.R. medRxiv Vol 20, 2020:July 06.
Dani M, Dirksen A, Taraborrelli P, Torocastro M, Panagopoulos D, Sutton R, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med (Lond). 2021;21(1):e63–7. https://doi.org/10.7861/clinmed.2020-0896. Epub 2020 Nov 26. PMID: 33243837; PMCID: PMC7850225.
Hellmuth J, Barnett TA, Asken BM, Kelly JD, Torres L, Stephens ML, et al. Persistent COVID-19-associated neurocognitive symptoms in non-hospitalized patients. J Neurovirol. 2021;27(1):191–5. https://doi.org/10.1007/s13365-021-00954-4. Epub 2021 Feb 2. PMID: 33528824; PMCID: PMC7852463.
Novak E. Neurological science. 2020.
Rahimi MM. BMJ Case Rep. 2021;14e:240178.
Hosey MM, Needham DM. Survivorship after COVID-19 ICU stay. Nat Rev Dis Primers. 2020;6(1):60. https://doi.org/10.1038/s41572-020-0201-1.PMID:32669623;PMCID:PMC7362322.
Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87(12):1196–201. https://doi.org/10.1016/j.mayocp.2012.10.013. PMID:23218087;PMCID:PMC3541923.
Rodríguez Y, Rojas M, Ramírez-Santana C, Acosta-Ampudia Y, Monsalve DM, Anaya JM. Autonomic symptoms following Zika virus infection. Clin Auton Res. 2018;28(2):211–4. https://doi.org/10.1007/s10286-018-0515-1. Epub 2018 Mar 1 PMID: 29497887.
Anaya JM, Rojas M, Salinas ML, Rodríguez Y, Roa G, Lozano M, et al. Post-COVID syndrome. A case series and comprehensive review. Autoimmun Rev. 2021;20(11):102947. https://doi.org/10.1016/j.autrev.2021.102947. Epub 2021 Sep 10. PMID: 34509649; PMCID: PMC8428988.
Freeman R, Abuzinadah AR, Gibbons C, Jones P, Miglis MG, Sinn DI. Orthostatic hypotension: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(11):1294–309. https://doi.org/10.1016/j.jacc.2018.05.079. PMID: 30190008.
Jardine DL, Wieling W, Brignole M, Lenders JWM, Sutton R, Stewart J. The pathophysiology of the vasovagal response. Heart Rhythm. 2018;15(6):921–9. https://doi.org/10.1016/j.hrthm.2017.12.013. Epub 2017 Dec 12. PMID: 29246828; PMCID: PMC5984661.
Konig MF, Powell M, Staedtke V, Bai RY, Thomas DL, Fischer N, et al. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. J Clin Invest. 2020;130(7):3345–7. https://doi.org/10.1172/JCI139642. PMID:32352407;PMCID:PMC7324164.
Fudim M, Qadri YJ, Ghadimi K, MacLeod DB, Molinger J, Piccini JP, et al. Implications for neuromodulation therapy to control inflammation and related organ dysfunction in COVID-19. J Cardiovasc Transl Res. 2020;13(6):894–9. https://doi.org/10.1007/s12265-020-10031-6. Epub 2020 May 26. PMID: 32458400; PMCID: PMC7250255.
Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2021;268(3):751–7. https://doi.org/10.1007/s00415-020-10108-x. Epub 2020 Jul 30. PMID: 32734353; PMCID: PMC7391231.
Fu Q, Vangundy TB, Shibata S, Auchus RJ, Williams GH, Levine BD. Exercise training versus propranolol in the treatment of the postural orthostatic tachycardia syndrome. Hypertension. 2011;58(2):167–75. https://doi.org/10.1161/HYPERTENSIONAHA.111.172262. Epub 2011 Jun 20. PMID: 21690484; PMCID: PMC3142863.
DePace NL, Vinik AI, Acosta CR, Eisen HJ, Colombo J. Oral vasoactive medications a summary of midodrine and droxidopa as applied to orthostatic dysfunction. Cardio Open. 2022;7(1):225–41.
Buoite Stella A, Furlanis G, Frezza NA, Valentinotti R, Ajcevic M, Manganotti P. Autonomic dysfunction in post-COVID patients with and without neurological symptoms: a prospective multidomain observational study. J Neurol. 2022;269(2):587–96. https://doi.org/10.1007/s00415-021-10735-y. Epub 2021 Aug 12. PMID: 34386903; PMCID: PMC8359764.
McMillan MT, Pan XQ, Smith AL, Newman DK, Weiss SR, Ruggieri MR Sr, et al. Coronavirus-induced demyelination of neural pathways triggers neurogenic bladder overactivity in a mouse model of multiple sclerosis. Am J Physiol Renal Physiol. 2014;307(5):F612–22. https://doi.org/10.1152/ajprenal.00151.2014. Epub 2014 Jul 9. PMID: 25007876; PMCID: PMC4154110.
Pourfridoni M, Pajokh M, Seyedi F. Bladder and bowel incontinence in COVID-19. J Med Virol. 2021;93(5):2609–10. https://doi.org/10.1002/jmv.26849. Epub 2021 Feb 19. PMID: 33543786; PMCID: PMC8014136.
Sansone A, Mollaioli D, Ciocca G, Limoncin E, Colonnello E, Vena W, et al. Addressing male sexual and reproductive health in the wake of COVID-19 outbreak. J Endocrinol Invest. 2021;44(2):223–31. https://doi.org/10.1007/s40618-020-01350-1. Epub 2020 Jul 13. PMID: 32661947; PMCID: PMC7355084.
Sansone A, Mollaioli D, Ciocca G, Colonnello E, Limoncin E, Balercia G, et al. “Mask up to keep it up”: preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021;9(4):1053–9. https://doi.org/10.1111/andr.13003 Epub 2021 Mar 30. PMID: 33742540; PMCID: PMC8250520.
Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833.e3. https://doi.org/10.1053/j.gastro.2020.02.055. Epub 2020 Mar 3. PMID: 32142773; PMCID: PMC7130181.
Domínguez-Varela IA, Rodríguez-Gutiérrez LA, Morales-Mancillas NR, Barrera-Sánchez M, Macías-Rodríguez Y, Valdez-García JE. COVID-19 and the eye: a review. Infect Dis (Lond). 2021;53(6):399–403. https://doi.org/10.1080/23744235.2021.1882697. Epub 2021 Feb 10 PMID: 33566704.
Karahan M, Demirtaş AA, Hazar L, Erdem S, Ava S, Dursun ME, et al. Autonomic dysfunction detection by an automatic pupillometer as a non-invasive test in patients recovered from COVID-19. Graefes Arch Clin Exp Ophthalmol. 2021;259(9):2821–6. https://doi.org/10.1007/s00417-021-05209-w. Epub 2021 Apr 27. PMID: 33907887; PMCID: PMC8078384.
Goodman BP, Khoury JA, Blair JE, Grill MF. COVID-19 dysautonomia. Front Neurol. 2021;13(12):624968. https://doi.org/10.3389/fneur.2021.624968. PMID:33927679;PMCID:PMC8076737.
Barizien N, Le Guen M, Russel S, et al. Clinical characterization of dysautonomia in long COVID-19 patients. Sci Rep. 2021;11:14042. https://doi.org/10.1038/s41598-021-93546-5.
Shouman K, Vanichkachorn G, Cheshire WP, Suarez MD, Shelly S, Lamotte GJ, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31(3):385–94. https://doi.org/10.1007/s10286-021-00803-8. Epub 2021 Apr 16. PMID: 33860871; PMCID: PMC8050227.
Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69(2):205–11. https://doi.org/10.1007/s12026-021-09185-5. Epub 2021 Mar 30. Erratum. In: Immunol Res. 2021 Apr 13;: PMID: 33786700; PMCID: PMC8009458.
Townsend L, Dowds J, O’Brien K, et al. Post–COVID-19 respiratory complications. Annals ATS. 2021;18(6).
Poenaru S, Abdallah SJ, Corrales-Medina V, Cowan J. COVID-19 and post-infectious myalgic encephalomyelitis/chronic fatigue syndrome: a narrative review. Ther Adv Infect Dis. 2021;20(8):20499361211009384. https://doi.org/10.1177/20499361211009385. PMID:33959278;PMCID:PMC8060761.
Sandler CX, Wyller VBB, Moss-Morris R, Buchwald D, Crawley E, Hautvast J, et al. Long COVID and post-infective fatigue syndrome: a review. Open Forum Infect Dis. 2021;8(10):ofab440. https://doi.org/10.1093/ofid/ofab440. PMID: 34631916; PMCID: PMC8496765.
Khatoon F, Prasad K, Kumar V. Neurological manifestations of COVID-19: available evidences and a new paradigm. J Neurovirol. 2020;26(5):619–30. https://doi.org/10.1007/s13365-020-00895-4. Epub 2020 Aug 24. PMID: 32839951; PMCID: PMC7444681.
Li W, Li M, Ou G. COVID-19, cilia, and smell. FEBS J. 2020;287(17):3672–6. https://doi.org/10.1111/febs.15491. Epub 2020 Aug 6. PMID: 32692465; PMCID: PMC7426555.
Kanwar D, Baig AM, Wasay M. Neurological manifestations of COVID-19. J Pak Med Assoc. 2020;70(Suppl 3(5)):S101–3. https://doi.org/10.5455/JPMA.20. PMID: 32515379.
Tetlow S, Segiet-Swiecicka A, O’Sullivan R, O’Halloran S, Kalb K, Brathwaite-Shirley C, et al. ACE inhibitors, angiotensin receptor blockers and endothelial injury in COVID-19. J Intern Med. 2021;289(5):688–99. https://doi.org/10.1111/joim.13202. Epub 2020 Dec 6. PMID: 33210357; PMCID: PMC7753609.
Wu J, Liang B, Chen C, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Commun. 2021;12:1813. https://doi.org/10.1038/s41467-021-22034-1.
Dey J, Alam MT, Chandra S, Gupta J, Ray U, Srivastava AK, Tripathi PP. Neuroinvasion of SARS-CoV-2 may play a role in the breakdown of the respiratory center of the brain. J Med Virol. 2021;93(3):1296–1303. https://doi.org/10.1002/jmv.26521. Epub 2020 Sep 28. PMID: 32964419.
Toljan K. Letter to the editor regarding the viewpoint “Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanism.” ACS Chem Neurosci. 2020;11(8):1192–4. https://doi.org/10.1021/acschemneuro.0c00174. Epub 2020 Apr 8. PMID: 32233443; PMCID: PMC7153046.
Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Gyebi GA, Batiha GE. Covid-19-induced dysautonomia: a menace of sympathetic storm. ASN Neuro. 2021;13:17590914211057636. https://doi.org/10.1177/17590914211057635. PMID: 34755562; PMCID: PMC8586167.
Hinduja A, Moutairou A, Calvet JH. Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiol Clin. 2021;51(2):193–6. https://doi.org/10.1016/j.neucli.2021.01.003. Epub 2021 Jan 26. PMID: 33551341; PMCID: PMC7835104.
Barizien N, Le Guen M, Russel S, Touche P, Huang F, Vallée A. Clinical characterization of dysautonomia in long COVID-19 patients. Sci Rep. 2021;11(1):14042. https://doi.org/10.1038/s41598-021-93546-5. PMID:34234251;PMCID:PMC8263555.
Becker RC. Autonomic dysfunction in SARS-COV-2 infection acute and long-term implications COVID-19 editor’s page series. J Thromb Thrombolysis. 2021;52(3):692–707. https://doi.org/10.1007/s11239-021-02549-6. Epub 2021 Aug 17. PMID: 34403043; PMCID: PMC8367772.
Crook H, Raza S, Nowell J, Young M, Edison P. Long COVID-mechanisms, risk factors, and management. BMJ. 2021;26(374):n1648. https://doi.org/10.1136/bmj.n1648. Erratum In: BMJ.2021 Aug3;374:n1944 PMID: 34312178.
Nalbandian A, Sehgal K, Gupta A. Post-acute COVID-19 syndrome. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01283-z.2021/03/22.
Mandal S, Barnett J, Brill SE. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2020. https://doi.org/10.1136/thoraxjnl-2020-215818.Nov10.
Adeghate EA, Eid N, Singh J. Mechanisms of COVID-19-induced heart failure: a short review. Heart Fail Rev. 2021;26(2):363–9. https://doi.org/10.1007/s10741-020-10037-x. Epub 2020 Nov 16. PMID: 33191474; PMCID: PMC7666972.
Paul BD, Lemle MD, Komaroff AL, Snyder SH. Redox imbalance links COVID-19 and myalgic encephalomyelitis/chronic fatigue syndrome. Proc Natl Acad Sci U S A. 2021;118(34):e2024358118. https://doi.org/10.1073/pnas.2024358118. PMID:34400495;PMCID:PMC8403932.
Sander LE, Garaude J. The mitochondrial respiratory chain: a metabolic rheostat of innate immune cell-mediated antibacterial responses. Mitochondrion. 2018;41:28–36. https://doi.org/10.1016/j.mito.2017.10.008. Epub 2017 Oct 18 PMID: 29054472.
Burtscher J, Cappellano G, Omori A, Koshiba T, Millet GP. Mitochondria: in the cross fire of SARS-CoV-2 and immunity. iScience. 2020;23(10):101631. https://doi.org/10.1016/j.isci.2020.101631. Epub 2020 Sep 29. PMID: 33015593; PMCID: PMC7524535.
• DePace NL, Colombo J. Autonomic and mitochondrial dysfunction in clinical diseases: diagnostic, prevention, and therapy. Springer Science + Business Media, New York, NY, 2019. This book provides the logic and evidence for the therapies recommended to treat the parasympathetic and sympathetic dysfunctions associated with Long-COVID syndrome.
Weill P, Plissonneau C, Legrand P, Rioux V, Thibault R. May omega-3 fatty acid dietary supplementation help reduce severe complications in COVID-19 patients? Biochimie. 2020;179:275–80. https://doi.org/10.1016/j.biochi.2020.09.003. Epub 2020 Sep 10. PMID: 32920170; PMCID: PMC7481803.
Adebayo A, Varzideh F, Wilson S, Gambardella J, Eacobacci M, Jankauskas SS, et al. l-Arginine and COVID-19: an update. Nutrients. 2021;13(11):3951. https://doi.org/10.3390/nu13113951. PMID:34836206;PMCID:PMC8619186.
Ziegler D, Gries F. Alpha-lipoic acid and the treatment of diabetic peripheral autonomic cardiac neuropathy. Diabetes. 1997;46(Suppl 2):S62–6.
Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29(11):2365–70.
Ametov AS, Barinov A, Dyck PJ, Hermann R, Kozlova N, Litchy WJ, et al. The sensory symptoms of diabetic polyneuropathy are improved with alphalipoic acid. The SYDNEY trial. Diabetes Care. 2003;26(3):770–6.
Ziegler D, Low PA, Litchy WJ, Boulton AJM, Vinik AI, Freeman R, et al. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy. Diabetes Care. 2011;34:2054–60.
Ogbonnaya S, Kaliaperumal C. Vagal nerve stimulator: evolving trends. J Nat Sci Biol Med. 2013;4(1):8–13. https://doi.org/10.4103/0976-9668.107254. PMID:23633829;PMCID:PMC3633308.
Squair JW, Berney M, Castro Jimenez M, Hankov N, Demesmaeker R, Amir S, et al. Implanted system for orthostatic hypotension in multiple-system atrophy. N Engl J Med. 2022;386(14):1339–44. https://doi.org/10.1056/NEJMoa2112809 PMID: 35388667.
Strain WD, Sherwood O, Banerjee A, Van der Togt V, Hishmeh L, Rossman J. The Impact of COVID Vaccination on Symptoms of Long COVID: An International Survey of People with Lived Experience of Long COVID. Vaccines (Basel). 2022;10(5):652. https://doi.org/10.3390/vaccines10050652. PMID: 35632408; PMCID: PMC9146071.
Bergwerk M, Gonen T, Lustig Y, Amit S, Lipsitch M, Cohen C, et al. COVID-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474–84. https://doi.org/10.1056/NEJMoa2109072. Epub 2021 Jul 28. PMID: 34320281; PMCID: PMC8362591.
Lambert NJ, Survivor Corps. COVID-19 “long hauler” symptoms survey report. Indiana University School of Medicine; 2020. https://dig.abclocal.go.com/wls/documents/2020/072720-wls-covid-symptom-study-doc.pdf. Accessed 1 Jul 2022.
Mancini DM, Brunjes DL, Lala A, Trivieri MG, Contreras JP, Natelson BH. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. 2021;9(12):927–37. https://doi.org/10.1016/j.jchf.2021.10.002. PMID:34857177;PMCID:PMC8629098.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Colombo reports the following: inventor of P&S Monitoring technology and officer of Physio PS, Inc. NeuroCardiology Research Corp. has no interest or involvement in this work. The other author has no disclosures to report.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Heart Failure
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
DePace, N.L., Colombo, J. Long-COVID Syndrome and the Cardiovascular System: A Review of Neurocardiologic Effects on Multiple Systems. Curr Cardiol Rep 24, 1711–1726 (2022). https://doi.org/10.1007/s11886-022-01786-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-022-01786-2