Abstract
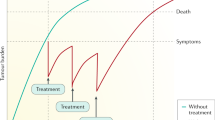
The positive results of sorafenib have unveiled a new direction of research in the management of hepatocellular carcinoma (HCC). Since then intensive efforts have been focused on development of novel management strategy to further improve the outcome for patients with HCC. Emerging data have suggested that tumor progression of HCC is driven by a number of deregulated signaling pathways and/or epigenetic mechanism. Thus much effort is dedicated to identification of novel agents targeting these dysregulated pathways. Combinations of targeted therapeutics and transarterial chemoembolization (TACE), or different systemic therapeutics also hold the promise to improve treatment outcome beyond sorafenib. This review aims to summarize the current status of clinical development of treatment in HCC. Perspectives on future direction of research will also be discussed.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76.
Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36.
Chan SL, Mo FK, Johnson PJ, et al. Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol. 2011;26:340–7.
Chan SL, Mo FK, Wong VW, et al. Use of antiviral therapy in surveillance: impact on outcome of hepatitis B-related hepatocellular carcinoma. Liver Int 2011 doi:10.1111/j.1478-3231.2011.02634.x.
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–42.
Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71.
Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford). 2005;7:35–41.
Chan AT, Kishi Y, Chan SL, Vauthey JN. Accomplishments in 2007 in the management of hepatobiliary cancers. Gastrointest Cancer Res. 2008;2:S25–31.
Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–8.
Chan SL, Mo FK, Johnson PJ, et al. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446–52.
Wilhelm SM, Carter C, Tang L, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–109.
Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43–9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59:561–74.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
•• Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. This phase III clinical trial showed that sorafenib could prolong overall survival of patients with advanced hepatocellular carcinoma in Asian population.
Toh HC, Chen PJ, Carr BI, et al. Linifanib phase II trial in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2010;28:15s. suppl; abstr 4038.
Koch I, Baron A, Roberts S, et al. Influence of hepatic dysfunction on safety, tolerability, and pharmacokinetics (PK) of PTK787/ZK 222584 in patients (Pts) with unresectable hepatocellular carcinoma (HCC). J Clin Oncol. 2005;23:341S-S.
Yau T, Chen PJ, Chan P, et al. Phase I dose-finding study of pazopanib in hepatocellular carcinoma: evaluation of early efficacy, pharmacokinetics, and pharmacodynamics. Clin Cancer Res;17:6914–23.
Bolondi L, Tak WY, Gasbarrini A, et al. Phase II safety study of the oral multikinase inhibitor regorafenib (BAY 73–4506) as second-line therapy in patients with hepatocellular carcinoma. Eur J Canc;47:S464-S.
Alberts SR, Morlan BW, Kim GP, et al. NCCTG phase II trial (N044J) of AZD2171 for patients with hepatocellular carcinoma (HCC)--Interim review of toxicity. ASCO Gastrointestinal Cancers Symposium 2007:Abstract number 186.
Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794–800.
Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–35.
Cheng A, Kang D, Lin J, et al. Phase III trial of sunitinib (Su) versus sorafenib (So) in advanced hepatocellular carcinoma (HCC). J Clin Oncol 2011;29:(suppl; abstr 4000).
Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603.
Cai ZW, Zhang Y, Borzilleri RM, et al. Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f] [1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215). J Med Chem. 2008;51:1976–80.
Park JW, Finn RS, Kim JS, et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011;17:1973–83.
Raoul JL, Finn RS, Kang YK, et al. An open-label phase II study of first- and second-line treatment with brivanib in patients with hepatocellular carcinoma (HCC). J Clin Oncol. 2009;27:15s. suppl; abstr 4577.
Laird AD, Vajkoczy P, Shawver LK, et al. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors. Cancer Res. 2000;60:4152–60.
Nakamura T, Ozawa S, Kitagawa Y, et al. Antiangiogenic agent SU6668 suppresses the tumor growth of xenografted A-431 cells. Oncol Rep. 2006;15:79–83.
Kanai F, Yoshida H, Tateishi R, et al. A phase I/II trial of the oral antiangiogenic agent TSU-68 in patients with advanced hepatocellular carcinoma. Cancer Chemother Pharmacol. 2011;67:315–24.
Lee SH, Lopes de Menezes D, Vora J, et al. In vivo target modulation and biological activity of CHIR-258, a multitargeted growth factor receptor kinase inhibitor, in colon cancer models. Clin Cancer Res. 2005;11:3633–41.
Huynh H, Chow PK, Tai WM, et al. Dovitinib demonstrates anti-tumor and anti-metastatic activities in xenograft models of hepatocellular carcinoma. J Hepatol 2011. doi:10.1016/j.jhep.2011.09.017.
Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–8.
Zhu AX, Finn RS, Mulcahy MF, et al. A phase II study of ramucirumab as first-line monotherapy in patients (pts) with advanced hepatocellular carcinoma (HCC). J Clin Oncol 2010;28:suppl; abstr 4083.
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57.
Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–17.
Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989–5005.
Morimitsu Y, Hsia CC, Kojiro M, Tabor E. Nodules of less-differentiated tumor within or adjacent to hepatocellular carcinoma: relative expression of transforming growth factor-alpha and its receptor in the different areas of tumor. Hum Pathol. 1995;26:1126–32.
Nalesnik MA, Lee RG, Carr BI. Transforming growth factor alpha (TGFalpha) in hepatocellular carcinomas and adjacent hepatic parenchyma. Hum Pathol. 1998;29:228–34.
Chung YH, Kim JA, Song BC, et al. Expression of transforming growth factor-alpha mRNA in livers of patients with chronic viral hepatitis and hepatocellular carcinoma. Cancer. 2000;89:977–82.
Philip PA, Mahoney MR, Allmer C, et al. Phase II study of Erlotinib (OSI-774) in patients with advanced hepatocellular cancer. J Clin Oncol. 2005;23:6657–63.
Thomas MB, Chadha R, Glover K, et al. Phase 2 study of erlotinib in patients with unresectable hepatocellular carcinoma. Cancer. 2007;110:1059–67.
Ramanathan RK, Belani CP, Singh DA, et al. A phase II study of lapatinib in patients with advanced biliary tree and hepatocellular cancer. Cancer Chemother Pharmacol. 2009;64:777–83.
Bekaii-Saab T, Markowitz J, Prescott N, et al. A multi-institutional phase II study of the efficacy and tolerability of lapatinib in patients with advanced hepatocellular carcinomas. Clin Cancer Res. 2009;15:5895–901.
Zhu AX, Stuart K, Blaszkowsky LS, et al. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:581–9.
Gruenwald Y, Wilkens L, Gebel M, et al. A phase II open-label study of cetuximab in unresectable hepatocellular carcinoma: final results. ASCO Annual Meeting Proceedings 2007;Part I. Vol 25, No. 18S 4598.
Pediaditakis P, Lopez-Talavera JC, Petersen B, et al. The processing and utilization of hepatocyte growth factor/scatter factor following partial hepatectomy in the rat. Hepatology. 2001;34:688–93.
Huh CG, Factor VM, Sanchez A, et al. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477–82.
Michieli P, Mazzone M, Basilico C, et al. Targeting the tumor and its microenvironment by a dual-function decoy Met receptor. Cancer Cell. 2004;6:61–73.
Comoglio PM, Trusolino L. Invasive growth: from development to metastasis. J Clin Invest. 2002;109:857–62.
Kiss A, Wang NJ, Xie JP, Thorgeirsson SS. Analysis of transforming growth factor (TGF)-alpha/epidermal growth factor receptor, hepatocyte growth Factor/c-met, TGF-beta receptor type II, and p53 expression in human hepatocellular carcinomas. Clin Cancer Res. 1997;3:1059–66.
Osada S, Kanematsu M, Imai H, Goshima S. Clinical significance of serum HGF and c-Met expression in tumor tissue for evaluation of properties and treatment of hepatocellular carcinoma. Hepatogastroenterology. 2008;55:544–9.
Ljubimova JY, Petrovic LM, Wilson SE, et al. Expression of HGF, its receptor c-met, c-myc, and albumin in cirrhotic and neoplastic human liver tissue. J Histochem Cytochem. 1997;45:79–87.
Okano J, Shiota G, Kawasaki H. Expression of hepatocyte growth factor (HGF) and HGF receptor (c-met) proteins in liver diseases: an immunohistochemical study. Liver. 1999;19:151–9.
Kaposi-Novak P, Lee JS, Gomez-Quiroz L, et al. Met-regulated expression signature defines a subset of human hepatocellular carcinomas with poor prognosis and aggressive phenotype. J Clin Invest. 2006;116:1582–95.
Ueki T, Fujimoto J, Suzuki T, et al. Expression of hepatocyte growth factor and its receptor, the c-met proto-oncogene, in hepatocellular carcinoma. Hepatology. 1997;25:619–23.
Ke AW, Shi GM, Zhou J, et al. Role of overexpression of CD151 and/or c-Met in predicting prognosis of hepatocellular carcinoma. Hepatology. 2009;49:491–503.
You H, Ding W, Dang H, et al. c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology. 2011;54:879–89.
Adjei AA, Schwartz B, Garmey E. Early clinical development of ARQ 197, a selective, non-ATP-competitive inhibitor targeting MET tyrosine kinase for the treatment of advanced cancers. Oncologist. 2011;16:788–99.
Zucali P, Santoro A, Rodriguez-Lope C, et al. Final results from ARQ 197–114: a phase Ib safety trial evaluating ARQ 197 in cirrhotic patients (pts) with hepatocellular carcinoma (HCC). J Clin Oncol 2010;28:suppl; abstr 4137.
Villanueva A, Chiang DY, Newell P, et al. Pivotal role of mTOR signaling in hepatocellular carcinoma. Gastroenterology. 2008;135:1972–83. 83 e1-11.
Sahin F, Kannangai R, Adegbola O, et al. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10:8421–5.
Zhu AX, Abrams TA, Miksad R, et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer. 2011;117:5094–102.
Huynh H, Nguyen TT, Chow KH, et al. Over-expression of the mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK in hepatocellular carcinoma: its role in tumor progression and apoptosis. BMC Gastroenterol. 2003;3:19.
O’Neil BH, Goff LW, Kauh JS, et al. Phase II study of the mitogen-activated protein kinase 1/2 inhibitor selumetinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2011;29:2350–6.
Balmanno K, Chell SD, Gillings AS, et al. Intrinsic resistance to the MEK1/2 inhibitor AZD6244 (ARRY-142886) is associated with weak ERK1/2 signalling and/or strong PI3K signalling in colorectal cancer cell lines. Int J Cancer. 2009;125:2332–41.
Ball DW, Jin N, Rosen DM, et al. Selective growth inhibition in BRAF mutant thyroid cancer by the mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244. J Clin Endocrinol Metab. 2007;92:4712–8.
Rodriguez-Paredes M, Esteller M. Cancer epigenetics reaches mainstream oncology. Nat Med. 2011;17:330–9.
Um TH, Kim H, Oh BK, et al. Aberrant CpG island hypermethylation in dysplastic nodules and early HCC of hepatitis B virus-related human multistep hepatocarcinogenesis. J Hepatol. 2011;54:939–47.
Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009;27:5410–7.
Mann BS, Johnson JR, He K, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13:2318–22.
• Ma BB, Sung F, Tao Q, et al. The preclinical activity of the histone deacetylase inhibitor PXD101 (belinostat) in hepatocellular carcinoma cell lines. Invest New Drugs. 2010;28:107–14. This study provided preclinical evidence of efficacy on a novel class of agent, histone deacetylase inhibitor, in HCC cell lines.
Carlisi D, Lauricella M, D’Anneo A, et al. The histone deacetylase inhibitor suberoylanilide hydroxamic acid sensitises human hepatocellular carcinoma cells to TRAIL-induced apoptosis by TRAIL-DISC activation. Eur J Cancer. 2009;45:2425–38.
Carlisi D, Vassallo B, Lauricella M, et al. Histone deacetylase inhibitors induce in human hepatoma HepG2 cells acetylation of p53 and histones in correlation with apoptotic effects. Int J Oncol. 2008;32:177–84.
Yeo W, Chung HC, Chan SL, et al. A phase II study of epigenetic therapy using belinostat for patients with unresectable hepatocellular carcinoma—a multicenter study of the Mayo Phase 2 Consortium (P2C) and the Cancer Therapeutics Research Group (CTRG). Eur J Canc;47:S470-S1.
Li X, Feng GS, Zheng CS, et al. Expression of plasma vascular endothelial growth factor in patients with hepatocellular carcinoma and effect of transcatheter arterial chemoembolization therapy on plasma vascular endothelial growth factor level. World J Gastroenterol. 2004;10:2878–82.
Wang B, Xu H, Gao ZQ, et al. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49:523–9.
Sergio A, Cristofori C, Cardin R, et al. Transcatheter Arterial Chemoembolization (TACE) in Hepatocellular Carcinoma (HCC): The Role of Angiogenesis and Invasiveness. Am J Gastroenterol 2008.
Jiang H, Meng Q, Tan H, et al. Antiangiogenic therapy enhances the efficacy of transcatheter arterial embolization for hepatocellular carcinomas. Int J Cancer. 2007;121:416–24.
Dufour JF, Hoppe H, Heim MH, et al. Continuous administration of sorafenib in combination with transarterial chemoembolization in patients with hepatocellular carcinoma: results of a phase I study. Oncologist;15:1198–204.
Kudo M, Imanaka K, Chida N, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Canc;47:2117–27.
• Pawlik TM, Reyes DK, Cosgrove D, et al. Phase II trial of Sorafenib combined with concurrent transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. J Clin Oncol. 2011;29:3960–7. This single-arm phase II study is the first clinical trial which demonstrated the potential efficacy and feasibility of combining targeted therapy and transarterial therapy for hepatocellular carcinoma.
Abou-Alfa GK. TACE and Sorafenib: a good marriage? J Clin Oncol. 2011;29:3949–52.
O’Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8.
Alberts SR, Reid JM, Morlan BW, et al. Gemcitabine and docetaxel for hepatocellular carcinoma: a Phase II North Central Cancer Treatment Group Clinical Trial. Am J Clin Oncol 2011; doi:10.1097/COC.0b013e318219863b.
Coriat R, Mir O, Cessot A, et al. Feasibility of oxaliplatin, 5-fluorouracil and leucovorin (FOLFOX-4) in cirrhotic or liver transplant patients: experience in a cohort of advanced hepatocellular carcinoma patients. Invest New Drugs 2011 doi:10.1007/s10637-010-9525-0.
• Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. Jama. 2010;304:2154–60. This paper reported on a randomized phase II clinical trial on the combination of chemotherapy and sorafenib in patients with advanced hepatocellular carcinoma. The study showed a promising survival benefit favoring the combination arm compared to doxorubicin alone.
Hsu CH, Shen YC, Lin ZZ, et al. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2011;53:126–31.
Yau T, Chan P, Cheung FY, et al. Phase II trial of sorafenib with capecitabine and oxaliplatin (SECOX) in patients with locally advanced or metastatic hepatocellular carcinoma. Ejc Supplements. 2009;7:20–1.
Thomas MB, Morris JS, Chadha R, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843–50.
Hsu CH, Yang TS, Hsu C, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981–6.
Philip PA, Mahoney MR, Holen KD, et al. Phase 2 study of bevacizumab plus erlotinib in patients with advanced hepatocellular cancer. Cancer 2011 doi:10.1002/cncr.26556.
van Malenstein H, van Pelt J, Verslype C. Molecular classification of hepatocellular carcinoma anno 2011. Eur J Cancer. 2011;47:1789–97.
Toh HC, Chen P, Knox JJ, et al. International phase 2 trial of ABT-869 in patients with advanced hepatocellular carcinoma (HCC). Ejc Supplements. 2009;7:366–7.
Chan SL, Yeo W. Targeted therapy of hepatocellular carcinoma: Present and future. J Gastroenterol Hepatol. 2012 doi:10.1111/j.1440-1746.2012.07096.x.
Disclosure
S. L. Chan: advisor for AstraZeneca and Novartis; T. Mok: advisor and received honorarium for speech engagement from AstraZeneca, Pfizer, Roche, Boehringer-Ingelheim, Eli Lilly, Eisai, AVEO, and Taiho; B. B.Y. Ma: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, S.L., Mok, T. & Ma, B.B.Y. Management of Hepatocellular Carcinoma: Beyond Sorafenib. Curr Oncol Rep 14, 257–266 (2012). https://doi.org/10.1007/s11912-012-0233-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-012-0233-0