Opinion statement
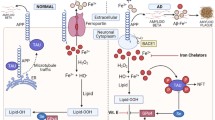
Medical treatment in Wilson’s disease includes chelators (d-penicillamine and trientine) or zinc salts that have to be maintain all the lifelong. This pharmacological treatment is categorised into two phases; the first being a de-coppering phase and the second a maintenance one. The best therapeutic approach remains controversial, as only a few non-controlled trials have compared these treatments. During the initial phase, progressive increase of chelators’ doses adjusted to exchangeable copper and urinary copper might help to avoid neurological deterioration. Liver transplantation is indicated in acute fulminant liver failure and decompensated cirrhosis; in cases of neurologic deterioration, it must be individually discussed. During the maintenance phase, the most important challenge is to obtain a good adherence to lifelong medical therapy. Neurodegenerative diseases that lead to a mislocalisation of iron can be caused by a culmination of localised overload (pro-oxidant siderosis) and localised deficiency (metabolic distress). A new therapeutic concept with conservative iron chelation rescues iron-overloaded neurons by scavenging labile iron and, by delivering this chelated metal to endogenous apo-transferrin, allows iron redistribution to avoid systemic loss of iron.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Fergusson JE. The heavy elements: chemistry, environmental impact and health effects. In: Fergusson JE, editor. Oxford: Pergamon Press; 1990.
Shaw CA, Tomljenovic L. Aluminum in the central nervous system (CNS): toxicity in humans and animals, vaccine adjuvants, and autoimmunity. Immunol Res. 2013;56(2–3):304–16.
Andersen O, Aaseth J. A review of pitfalls and progress in chelation treatment of metal poisonings. J Trace Elem Med Biol. 2016.
Sethi PK, Khandelwal D. Cadmium exposure: health hazards of silver cottage industry in developing countries. J Med Toxicol. 2006;2(1):14–5.
Brodkin E et al. Lead and mercury exposures: interpretation and action. CMAJ. 2007;176(1):59–63.
Clarkson TW, Magos L, Myers GJ. The toxicology of mercury—current exposures and clinical manifestations. N Engl J Med. 2003;349(18):1731–7.
Krebs N et al. Assessment of trace elements in human brain using inductively coupled plasma mass spectrometry. J Trace Elem Med Biol. 2014;28(1):1–7.
Choi BS, Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2009;1248:14–21.
Monnot AD et al. Regulation of brain copper homeostasis by the brain barrier systems: effects of Fe-overload and Fe-deficiency. Toxicol Appl Pharmacol. 2011;256(3):249–57.
Lutsenko S, Bhattacharjee A, Hubbard AL. Copper handling machinery of the brain. Metallomics. 2010;2(9):596–608. Summary of the current knowledge about cerebral copper levels and actions. Fine description of the molecules involved in maintaining copper homeostasis in the brain.
Ahuja A et al. Copper mediated neurological disorder: visions into amyotrophic lateral sclerosis, Alzheimer and Menkes disease. J Trace Elem Med Biol. 2015;29:11–23.
Zischka H, Lichtmannegger J. Pathological mitochondrial copper overload in livers of Wilson’s disease patients and related animal models. Ann N Y Acad Sci. 2014;1315:6–15.
Woimant F, Trocello JM. Disorders of heavy metals. Handb Clin Neurol. 2014;120:851–64. Insightful review of the current clinical, diagnostic and treatment challenges in heavy metals disorders.
Coffey AJ et al. A genetic study of Wilson’s disease in the United Kingdom. Brain. 2013;136(Pt 5):1476–87.
Trocello JM et al. Extensive striatal, cortical, and white matter brain MRI abnormalities in Wilson disease. Neurology. 2013;81(17):1557.
Trocello JM et al. Corpus callosum abnormalities in Wilson’s disease. J Neurol Neurosurg Psychiatry. 2011;82(10):1119–21.
El Balkhi S et al. Relative exchangeable copper: a new highly sensitive and highly specific biomarker for Wilson’s disease diagnosis. Clin Chim Acta. 2011;412(23–24):2254–60.
Bandmann O, Weiss KH, Kaler SG. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015;14(1):103–13. A comprehensive review of Wilson’s disease including its phenomenology, pathophysiology, imaging findings and treatment.
Merle U et al. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: a cohort study. Gut. 2007;56(1):115–20.
Weiss KH et al. Efficacy and safety of oral chelators in treatment of patients with Wilson disease. Clin Gastroenterol Hepatol. 2013;11(8):1028–35. e1-2.
Czlonkowska A et al. D-penicillamine versus zinc sulfate as first-line therapy for Wilson’s disease. Eur J Neurol. 2014;21(4):599–606.
Weiss KH et al. Zinc monotherapy is not as effective as chelating agents in treatment of Wilson disease. Gastroenterology. 2011;140(4):1189–98. e1.
Brewer GJ et al. Treatment of Wilson’s disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine. Transl Res. 2009;154(2):70–7.
Guillaud O et al. Long term results of liver transplantation for Wilson’s disease: experience in France. J Hepatol. 2014;60(3):579–89.
Medici V et al. Liver transplantation for Wilson’s disease: the burden of neurological and psychiatric disorders. Liver Transpl. 2005;11(9):1056–63.
Delangle P, Mintz E. Chelation therapy in Wilson’s disease: from D-penicillamine to the design of selective bioinspired intracellular Cu(I) chelators. Dalton Trans. 2012;41(21):6359–70.
Roybal JL et al. Early gestational gene transfer with targeted ATP7B expression in the liver improves phenotype in a murine model of Wilson’s disease. Gene Ther. 2012;19(11):1085–94.
Kaler SG. Translational research investigations on ATP7A: an important human copper ATPase. Ann N Y Acad Sci. 2014;1314:64–8. A general and comprehensive review of disorders due to ATP7A dysfunctions.
Donsante A et al. ATP7A gene addition to the choroid plexus results in long-term rescue of the lethal copper transport defect in a Menkes disease mouse model. Mol Ther. 2011;19(12):2114–23.
Kaler SG. Inborn errors of copper metabolism. Handb Clin Neurol. 2013;113:1745–54.
Martinelli D et al. MEDNIK syndrome: a novel defect of copper metabolism treatable by zinc acetate therapy. Brain. 2013;136(Pt 3):872–81.
Huppke P et al. Mutations in SLC33A1 cause a lethal autosomal-recessive disorder with congenital cataracts, hearing loss, and low serum copper and ceruloplasmin. Am J Hum Genet. 2012;90(1):61–8.
Teodoro T et al. Recovery after copper-deficiency myeloneuropathy in Wilson’s disease. J Neurol. 2013;260(7):1917–8.
Prohaska JR. Impact of copper deficiency in humans. Ann N Y Acad Sci. 2014;1314:1–5.
Halfdanarson TR et al. Hematological manifestations of copper deficiency: a retrospective review. Eur J Haematol. 2008;80(6):523–31.
Bremner I. Manifestations of copper excess. Am J Clin Nutr. 1998;67(5 Suppl):1069S–73.
Fleming RE, Ponka P. Iron overload in human disease. N Engl J Med. 2012;366(4):348–59.
Camaschella C. Hereditary sideroblastic anemias: pathophysiology, diagnosis, and treatment. Semin Hematol. 2009;46(4):371–7.
Cairo G, Recalcati S. Iron-regulatory proteins: molecular biology and pathophysiological implications. Expert Rev Mol Med. 2007;9(33):1–13.
Sohn YS et al. Redistribution of accumulated cell iron: a modality of chelation with therapeutic implications. Blood. 2008;111(3):1690–9.
Rouault TA. Iron metabolism in the CNS: implications for neurodegenerative diseases. Nat Rev Neurosci. 2013;14(8):551–64.
Rouault TA. Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Dis Model Mech. 2012;5(2):155–64.
Guzman JN et al. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468(7324):696–700.
Milusheva E et al. Increased sensitivity of striatal dopamine release to H2O2 upon chronic rotenone treatment. Free Radic Biol Med. 2005;39(1):133–42.
Zecca L et al. Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci. 2004;5(11):863–73.
Hutchinson C. A review of iron studies in overweight and obese children and adolescents: a double burden in the young? Eur J Nutr. 2016.
Cabantchik ZI et al. Regional siderosis: a new challenge for iron chelation therapy. Front Pharmacol. 2013;4:167.
Devos D et al. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal. 2014;21(2):195–210. Establishes the therapeutic features of chelation modality in PD translational models and in pilot clinical trials.
Levi S, Finazzi D. Neurodegeneration with brain iron accumulation: update on pathogenic mechanisms. Front Pharmacol. 2014;5:99.
Zorzi G et al. Therapeutic advances in neurodegeneration with brain iron accumulation. Semin Pediatr Neurol. 2012;19(2):82–6.
Zorzi G et al. Iron-related MRI images in patients with pantothenate kinase-associated neurodegeneration (PKAN) treated with deferiprone: results of a phase II pilot trial. Mov Disord. 2011;26(9):1756–9.
Abbruzzese G et al. A pilot trial of deferiprone for neurodegeneration with brain iron accumulation. Haematologica. 2011;96(11):1708–11.
Cossu G et al. Efficacy and safety of deferiprone for the treatment of pantothenate kinase-associated neurodegeneration (PKAN) and neurodegeneration with brain iron accumulation (NBIA): results from a four years follow-up. Parkinsonism Relat Disord. 2014;20(6):651–4.
Connor JR et al. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res. 1990;27(4):595–611.
Dexter DT et al. Increased nigral iron content in postmortem parkinsonian brain. Lancet. 1987;2(8569):1219–20.
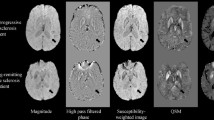
Du G et al. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov Disord. 2011;26(9):1627–32.
Peran P et al. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain. 2010;133(11):3423–33.
Ducic T et al. X-ray fluorescence analysis of iron and manganese distribution in primary dopaminergic neurons. J Neurochem. 2013;124(2):250–61.
Groger A, Berg D. Does structural neuroimaging reveal a disturbance of iron metabolism in Parkinson’s disease? Implications from MRI and TCS studies. J Neural Transm (Vienna). 2012;119(12):1523–8.
Wallis LI et al. MRI assessment of basal ganglia iron deposition in Parkinson’s disease. J Magn Reson Imaging. 2008;28(5):1061–7.
Przedborski S, Vila M. The 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model: a tool to explore the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci. 2003;991:189–98.
Lhermitte J, Kraus WM, McAlpine D. Original papers: on the occurrence of abnormal deposits of iron in the brain in parkinsonism with special reference to its localisation. J Neurol Psychopathol. 1924;5(19):195–208.
Berg D. Disturbance of iron metabolism as a contributing factor to SN hyperechogenicity in Parkinson’s disease: implications for idiopathic and monogenetic forms. Neurochem Res. 2007;32(10):1646–54.
Lotharius J, Brundin P. Pathogenesis of Parkinson’s disease: dopamine, vesicles and alpha-synuclein. Nat Rev Neurosci. 2002;3(12):932–42.
Hare DJ, Double KL. Iron and dopamine: a toxic couple. Brain. 2016.
Moos T et al. Iron trafficking inside the brain. J Neurochem. 2007;103(5):1730–40.
Zucca FA, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol. 2015.
Wong BX, Duce JA. The iron regulatory capability of the major protein participants in prevalent neurodegenerative disorders. Front Pharmacol. 2014;5:81.
Ayton S et al. Ceruloplasmin dysfunction and therapeutic potential for Parkinson disease. Ann Neurol. 2013;73(4):554–9.
Lei P et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18(2):291–5.
Kakhlon O et al. Iron redistribution as a therapeutic strategy for treating diseases of localized iron accumulation. Can J Physiol Pharmacol. 2010;88(3):187–96.
Chamberlain R et al. Comparison of amyloid plaque contrast generated by T2-weighted, T2*-weighted, and susceptibility-weighted imaging methods in transgenic mouse models of Alzheimer’s disease. Magn Reson Med. 2009;61(5):1158–64.
Yao B et al. Susceptibility contrast in high field MRI of human brain as a function of tissue iron content. Neuroimage. 2009;44(4):1259–66.
Zeineh MM et al. Activated iron-containing microglia in the human hippocampus identified by magnetic resonance imaging in Alzheimer disease. Neurobiol Aging. 2015;36(9):2483–500.
Quintana C et al. Study of the localization of iron, ferritin, and hemosiderin in Alzheimer’s disease hippocampus by analytical microscopy at the subcellular level. J Struct Biol. 2006;153(1):42–54.
Crespo AC et al. Genetic and biochemical markers in patients with Alzheimer’s disease support a concerted systemic iron homeostasis dysregulation. Neurobiol Aging. 2014;35(4):777–85.
Ayton S et al. Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun. 2015;6:6760. Identifies that elevated brain iron adversely impacts on AD progression and introduces the concept that brain iron elevation is a possible mechanism for APOEε44 being the major genetic risk factor for AD.
Xu H et al. Iron regulates apolipoprotein E expression and secretion in neurons and astrocytes. J Alzheimers Dis. 2016;51(2):471–87.
Kauwe JS et al. Suggestive synergy between genetic variants in TF and HFE as risk factors for Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(4):955–9.
Rogers JT et al. An iron-responsive element type II in the 5′-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem. 2002;277(47):45518–28.
Duce JA et al. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell. 2010;142(6):857–67.
Wong BX et al. Beta-amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS One. 2014;9(12):e114174. Confirms that APP stabilises ferroportin on the neuronal surface and facilitates iron efflux despite lacking ferroxidase activity.
Wan L et al. Overexpression of human wild-type amyloid-beta protein precursor decreases the iron content and increases the oxidative stress of neuroblastoma SH-SY5Y cells. J Alzheimers Dis. 2012;30(3):523–30.
Needham BE, Ciccotosto GD, Cappai R. Combined deletions of amyloid precursor protein and amyloid precursor-like protein 2 reveal different effects on mouse brain metal homeostasis. Metallomics. 2014;6(3):598–603.
McCarthy RC, Park YH, Kosman DJ. sAPP modulates iron efflux from brain microvascular endothelial cells by stabilizing the ferrous iron exporter ferroportin. EMBO Rep. 2014;15(7):809–15.
Wan L et al. Beta-amyloid peptide increases levels of iron content and oxidative stress in human cell and Caenorhabditis elegans models of Alzheimer disease. Free Radic Biol Med. 2011;50(1):122–9.
Guo C et al. Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34(2):562–75.
Su XW et al. Serum ferritin is elevated in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(1–2):102–7.
Veyrat-Durebex C et al. Iron metabolism disturbance in a French cohort of ALS patients. Biomed Res Int. 2014;2014:485723. This is the first study to show a higher concentration of serum iron in ALS patients, strengthening the involvement of deregulated iron metabolism in ALS.
Nadjar Y et al. Elevated serum ferritin is associated with reduced survival in amyotrophic lateral sclerosis. PLoS One. 2012;7(9):e45034.
Ikeda K et al. Relationships between disease progression and serum levels of lipid, urate, creatinine and ferritin in Japanese patients with amyotrophic lateral sclerosis: a cross-sectional study. Intern Med. 2012;51(12):1501–8.
Ignjatovic A et al. Inappropriately chelated iron in the cerebrospinal fluid of amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler. 2012;13(4):357–62.
Langkammer C et al. Mapping of iron deposition in conjunction with assessment of nerve fiber tract integrity in amyotrophic lateral sclerosis. J Magn Reson Imaging. 2010;31(6):1339–45.
Kwan JY et al. Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One. 2012;7(4):e35241.
Jeong SY et al. Dysregulation of iron homeostasis in the CNS contributes to disease progression in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2009;29(3):610–9.
Wang Q et al. Prevention of motor neuron degeneration by novel iron chelators in SOD1(G93A) transgenic mice of amyotrophic lateral sclerosis. Neurodegener Dis. 2011;8(5):310–21.
Haraguchi T et al. Coexistence of TDP-43 and tau pathology in neurodegeneration with brain iron accumulation type 1 (NBIA-1, formerly Hallervorden-Spatz syndrome). Neuropathology. 2011;31(5):531–9.
Martinez-Finley EJ et al. Manganese neurotoxicity and the role of reactive oxygen species. Free Radic Biol Med. 2013;62:65–75.
Guilarte TR. Manganese neurotoxicity: new perspectives from behavioral, neuroimaging, and neuropathological studies in humans and non-human primates. Front Aging Neurosci. 2013;5:23.
Klos KJ et al. Brain metal concentrations in chronic liver failure patients with pallidal T1 MRI hyperintensity. Neurology. 2006;67(11):1984–9.
Acknowledgments
JD is supported by the European Research Council and Australian National Health & Medical Research Council (NHMRC) (#1061587). DD was supported by the French Ministry of Health for funding PHRC grants, the Association of patients ARSLA, the French Parkinson’s Disease Association, DN2M and the European Union (Program Horizon 2020 PHC13-2014/2015 N°633190). Apopharma has provided DFP and placebo formulations for the clinical trials reported in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Aurélia Poujois, Jean-Christophe Devedjian, Pascal Chaine, France Woimant, and James A. Duce each declare no potential conflicts of interest.
David Devos served on the Scientific Advisory Board for Novartis, Aguettant, Orkyn, Alzprotect, Apopharma. He has received various honoraria from pharmaceutical companies for consultancy and lectures on Parkinson’s disease at symposia.
Caroline Moreau has served on the Scientific Advisory Board for Aguettant and Abbvie. She has received various honoraria from pharmaceutical companies for consultancy and lectures on Parkinson’s disease at symposia.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors. This article reports results from studies performed in animals and humans.
Additional information
This article is part of the Topical Collection on Neurologic Manifestations of Systemic Disease
Rights and permissions
About this article
Cite this article
Poujois, A., Devedjian, JC., Moreau, C. et al. Bioavailable Trace Metals in Neurological Diseases. Curr Treat Options Neurol 18, 46 (2016). https://doi.org/10.1007/s11940-016-0426-1
Published:
DOI: https://doi.org/10.1007/s11940-016-0426-1