Abstract
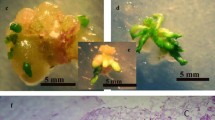
Banana is an important tropical fruit worldwide. Parthenocarpy and female sterility made it impossible to improve banana varieties through common hybridization. Genetic transformation for banana improvement is imperative. But the low rate that banana embryogenic callus was induced made the transformation cannot be performed in many laboratories. Finding ways to promote banana somatic embryogenesis is critical for banana genetic transformation. After tobacco arabinogalactan protein gene NtEPc was transformed into Escherichia coli (DE3), the recombinant protein was purified and filter-sterilized. A series of the sterilized protein was added into tissue culture medium. It was found that the number of banana immature male flowers developing embryogenic calli increased significantly in the presence of NtEPc protein compared with the effect of the control medium. Among the treatments, explants cultured on medium containing 10 mg/l of NtEPc protein had the highest chance to develop embryogenic calli. The percentage of lines that developed embryogenic calli on this medium was about 12.5 %. These demonstrated that NtEPc protein can be used to promote banana embryogenesis. This is the first paper that reported that foreign arabinogalactan protein (AGP) could be used to improve banana somatic embryogenesis.





Similar content being viewed by others
References
Chen, Y. F., Dai, X. M., Gong, Q., Huang, X., Xiao, W., Zhao, J. T., & Huang, X. L. (2011) Non-conventional breeding of banana (Musa spp.). In Jiang ZY, Yi GJ, Molina A, Bergh IV. Proceedings of the international ISHS-Promusa symposium on global perspectives on Asian challenges. Guangzhou, China. page 39–46.
Cote, F. X., Domergue, R., Monmarson, S., Schwendiman, J., Teisson, C., & Escalant, J. V. (1996). Embryogenic cell suspensions from the male flower of Musa AAA cv. Grand nain. Physiologia Plantarum, 97, 285–290.
Egertsdotter, U., & von Arnold, S. (1995). Importance of arabinogalactan proteins for the development of somatic embryos of Norway spruce (Picea abies). Physiologia Plantarum, 93, 334–345.
Escalant, J. V., Teisson, C., & Cote, F. X. (1994). Amplified somatic embryogenesis from male flowers of triploid banana and plantain cultivars (Musa spp.). In Vitro Cell Biology Development, 30, 181–186.
Hu, Z., Chen, H., Wu, G., Zhang, X., Jin, W., Xia, Z., & Chen, J. (2003). Establishment of suspension cell lines and high frequency regeneration of green plants in tall fescue. Acta Prataculturae Sinica, 12(3), 95–99.
Huang, X., Huang, X. L., Xiao, W., Zhao, J. T., Dai, X. M., Chen, Y. F., & Li, X. J. (2007). Highly efficient Agrobacterium-mediated transformation of embryogenic cell suspensions of Musa acuminate cv. Mas (AA) via a liquid co-cultivation system. Plant Cell Reports, 26, 1755–1762.
Khanna, H., Paul, J., Harding, R., Dickman, M., & Dale, J. (2007). Inhibition of Agrobacterium-induced cell death by antiapoptotic gene expression leads to very high transformation efficiency of banana. Molecular Plant-Microbe Interactions, 20(9), 1048–1054.
Kreuger, M., & van Holst, G. J. (1993). Arabinogalactan proteins are essential in somatic embryogenesis of Daucus carota L. Planta, 189, 243–248.
Kyo, M., Miyatake, H., Mamezuka, K., & Amagata, K. (2000). Cloning of cDNA encoding NtEPc, a marker protein for the embryogenic dedifferentiation of immature tobacco pollen grains cultured in vitro. Plant and Cell Physiology, 41(2), 129–137.
Lin, S. L. (2011). Concise review: deciphering the mechanism behind induced pluripotent stem cell generation. Stem Cells, 29, 1645–1649.
Lin, S. L., Chang, D., Lin, C. H., Ying, S. Y., Leu, D., & Wu, D. T. (2011). Regulation of somatic cell reprogramming through inducible mir-302 expression. Nucleic Acids Research, 39, 1054–1065.
Lough, T. J., & Lucas, W. J. (2006). Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annual Review of Plant Biology, 57, 203–232.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15(3), 473–497.
Namanya, P., Magambo, S. M., Mutumba, G., & Tushemereirwe, W. (2004). Somatic embryogenesis from immature male inflorescences of east African highland banana CV ‘Nakyetengu’. African Crop Science Journal, 12(1), 43–49.
Paungfoo-Lonhienne, C., Lonhienne, T. G., Rentsch, D., Robinson, N., Christie, M., Webb, R. I., Gamage, H. K., Carroll, B. J., Schenk, P. M., & Schmidt, S. (2008). Plants can use protein as a nitrogen source without assistance from other organisms. PNAS, 105(11), 4524–4529.
Poon, S., Heath, R. L., & Clarke, A. Z. (2012). A chimeric arabinogalactan protein promotes somatic embryogenesis in cotton cell culture. Plant Physiology, 160, 684–695.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
Schultz, C. J., Johnson, K. L., Currie, G., & Bacic, A. (2000). The classical arabinogalactan protein gene family of Arabidopsis. The Plant Cell, 12(9), 1751–1768.
Strosse, H., Domergue, R., Panis, B., Escalant, J., & Cote, F. (2001). Banana and plantain embryogenic cell suspensions. INIBAP Technical Guidelines 8: 1–31 Montpellier Cedex, France.
Vuylsteke, D. R., Crouch, J. H., Pellegrinschi, A., & Thottapilly, G. (1998). The biotechnology case history for Musa. Drew RA (Ed.). Proceedings of international symposium on biotechnology, tropical and sub-tropical species.
Vuylsteke, D., Swennen, R., & Ortiz, R. (1993). Development and performance of black sigatoka-resistant tetraploid hybrids of plantain (Musa spp., AAB group). Euphyta, 65, 33–42.
Yang, Y., Jian, Y., & Zheng, Y. (1999). Copper enhances plant regeneration in callus culture of rice. Chinese Journal Rice Science, 13(2), 95–98.
Yu, S., Zhu, Y., Yu, Y., Zhang, R., & Zhang, D. (2001). Elementary exploration about the cultural procedure for rapidly establishing cell embryogenic suspension clones. Journal of Huazhong Agricultural University, 20(4), 325–328.
Acknowledgments
This work was supported by National Science Foundation of China (No. 31371696 and No. 41201309), Fundamental Scientific Research Funds for CATAS-TCGRI (No. 1630032013022) and Science Foundation of Hainan Province (No. 313065).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shu, H., Xu, L., Li, Z. et al. Tobacco Arabinogalactan Protein NtEPc Can Promote Banana (Musa AAA) Somatic Embryogenesis. Appl Biochem Biotechnol 174, 2818–2826 (2014). https://doi.org/10.1007/s12010-014-1228-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-014-1228-0