Abstract
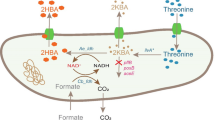
To increase the biocatalytic activity of Escherichia coli CCZU-T15 whole cells, choline chloride/glycerol ([ChCl][Gly]) was firstly used as biocompatible solvent for the effective biotransformation of ethyl 4-chloro-3-oxobutanoate (COBE) into ethyl (S)-4-chloro-3-hydroxybutanoate [(S)-CHBE]. Furthermore, L-glutamine (150 mM) was added into [ChCl][Gly]–water ([ChCl][Gly] 12.5 vol%, pH 6.5) media instead of NAD+ for increasing the biocatalytic efficiency. To further improve the biosynthesis of (S)-CHBE (>99 % e.e.) by E. coli CCZU-T15 whole cells, Tween-80 (7.5 mM) was also added into this reaction media, and (S)-CHBE (>9 % e.e.) could be effectively synthesized from 2000 and 3000 mM COBE in the yields of 100 and 93.0 % by whole cells of recombinant E. coli CCZU-T15, respectively. TEM image indicated that the cell membrane was permeabilized and lost its integrity and when the cell was exposed to [ChCl][Gly]–water media with Tween-80. Clearly, this bioprocess has high potential for the effective biosynthesis of (S)-CHBE (>99 % e.e.).








Similar content being viewed by others
References
Sun, J., Huang, J. H., Ding, X. Z., & Wang, P. (2016). Efficient enantioselective biocatalytic production of a chiral intermediate of Sitagliptin by a newly filamentous fungus isolate. Applied Biochemistry and Biotechnology. doi:10.1007/s12010-016-2125-5.
Zhang, R., Xu, Y., Geng, Y. W., Wang, S. S., Sun, W. Y., & Xiao, R. (2010). Improved production of (R)-1-phenyl-1,2-ethanediol by a codon-optimized R-specific carbonyl reductase from Candida parapsilosis in Escherichia coli. Applied Biochemistry and Biotechnology, 160, 868–878.
Ema, T., Yagasaki, H., Okita, N., Takeda, M., & Sakai, T. (2006). Asymmetric reduction of ketones using recombinant E. coli cells that produce a versatile carbonyl reductase with high enantioselectivity and broad substrate specificity. Tetrahedron, 62, 6143–6149.
Ema, T., Ide, S., Okita, N., & Sakai, T. (2008). Highly efficient chemoenzymatic synthesis of methyl (R)-o-chloromandelate, a key intermediate for clopidogrel, via asymmetric reduction with recombinant Escherichia coli. Advanced Synthesis and Catalysis, 350, 2039–2044.
He, Y. C., Zhang, D. P., Tao, Z. C., Lu, Y., Ding, Y., Liu, F., Zhu, Z. Z., Rui, H., Zheng, G. W., & Zhang, X. (2015). Improved biosynthesis of ethyl (S)-4-chloro-3-hydroxybutanoate by adding L-glutamine plus glycine instead of NAD+ in β-cyclodextrin-water system. Bioresource Technology, 182, 98–102.
He, Y. C., Tao, Z. C., Di, J. H., Chen, L., Zhang, L. B., Zhang, D. P., Chong, G. G., Liu, F., Ding, Y., Jiang, C. X., & Ma, C. L. (2016). Effective asymmetric bioreduction of ethyl 4-chloro-3-oxobutanoate to ethyl (R)-4-chloro-3-hydroxybutanoate by recombinant E. coli CCZU-A13 in [Bmim]PF6-hydrolyzate media. Bioresource Technology, 214, 411–418.
Cao, H., Mi, L., Ye, Q., Zhang, G. L., Yan, M., Wang, Y., Zhang, Y. Y., Li, X. M., Xu, L., Xiong, J., Ouyang, P. K., & Ying, H. J. (2011). Purification and characterization of a novel NADH-dependent carbonyl reductase from Pichia stipitis involved in biosynthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate. Bioresource Technology, 102, 1733–1739.
He, Y. C., Yang, Z. X., Zhang, D. P., Tao, Z. C., Chen, C., Chen, Y. T., Guo, F., Xu, J. H., Huang, L., Chen, R. J., & Ma, X. F. (2014). Biosynthesis of ethyl (S)-4-chloro-3-hydroxybutanoate by NADH-dependent reductase from E. coli CCZU-Y10 discovered by genome data mining using mannitol as cosubstrate. Applied Biochemistry Biotechnology, 173, 2042–2053.
Kizaki, N., Yasohara, Y., Hasegawa, J., Wada, M., Kataoka, M., & Shimizu, S. (2001). Synthesis of optically pure ethyl (S)-4-chloro-3-hydroxybutanoate by Escherichia coli transformant cells coexpressing the carbonyl reductase and glucose dehydrogenase genes. Applied Microbiology and Biotechnology, 55, 590–595.
Yamamoto, H., Mitsuhashi, K., Kimoto, N., Matsuyama, A., Esaki, N., & Kobayashi, Y. (2004). A novel NADH-dependent carbonyl reductase from Kluyveromyces aestuarii and comparison of NADH-regeneration system for the synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate. Bioscience, Biotechnology, and Biochemistry, 68, 638–649.
Ye, Q., Yan, M., Yao, Z., Xu, L., Cao, H., Li, Z. J., Chen, Y., Li, S. Y., Bai, J. X., Xiong, J., Ying, H. J., & Ouyang, P. K. (2009). A new member of the short-chain dehydrogenases/reductases superfamily: purification, characterization and substrate specificity of a recombinant carbonyl reductase from Pichia stipitis. Bioresource Technology, 100, 6022–6027.
Yu, M. A., Wei, Y. M., Zhao, L., Jiang, L., Zhu, X. B., & Qi, W. (2007). Bioconversion of ethyl 4-chloro-3-oxobutanoate by permeabilized fresh brewer’s yeast cells in the presence of allyl bromide. Journal of Industrial Microbiology & Biotechnology, 34, 151–156.
Wang, L. J., Li, C. X., Ni, Y., Zhang, J., Liu, X., & Xu, J. H. (2011). Highly efficient synthesis of chiral alcohols with a novel NADH-dependent reductase from Streptomyces coelicolor. Bioresource Technology, 102, 7023–7028.
Wang, Q. Y., Shen, L. H., Ye, T. T., Cao, D., Chen, R., Pei, X. L., Xie, T., Li, Y., Gong, W. B., & Yin, X. P. (2012). Overexpression and characterization of a novel (S)-specific extended short-chain dehydrogenase/reductase from Candida parapsilosis. Bioresource Technology, 123, 690–694.
Ye, Q., Cao, H., Mi, L., Yan, M., Wang, Y., He, Q. T., Li, J., Xu, L., Chen, Y. J., Xiong, J., Ouyang, P. K., & Ying, H. J. (2010). Biosynthesis of (S)-4-chloro-3-hydroxybutanoate ethyl using Escherichia coli co-expressing a novel NADH-dependent carbonyl and a glucose dehydrogenase. Bioresource Technology, 101, 8911–8914.
He, Y. C., Tao, Z. C., Zhang, X., Yang, Z. X., & Xu, J. H. (2014). Highly efficient synthesis of ethyl (S)-4-chloro-3-hydroxybutanoate and its derivatives by a robust NADH dependent reductase from E. coli CCZU-K14. Bioresource Technology, 161, 461–464.
Ishige, T., Honda, K., & Shimizu, S. (2005). Whole organism biocatalysis. Current Opinion in Chemical Biology, 9, 174–180.
Ni, Y., Sun, Y. N., Li, H. D., Zhou, J. Y., & Sun, Z. H. (2013). Scalable biocatalytic synthesis of optically pure ethyl (R)-2-hydroxy-4-phenylbutyrate using a recombinant E. coli with high catalyst yield. Journal of Biotechnology, 168, 493–498.
Nie, Y., Xu, Y., Hu, Q. S., & Xiao, R. (2009). A new strategy to improve the efficiency and sustainability of Candida parapsilosis catalyzing deracemization of (R,S)-1-phenyl-1,2-ethanediol under non-growing conditions: increase of NADPH availability. Journal of Microbiology and Biotechnology, 19, 65–71.
Li, N., Zhang, Y. Y., Ye, Q., Zhang, Y. Z., Chen, Y., Chen, X. C., Wu, J. L., Bai, J. X., Xie, J. J., & Ying, H. J. (2012). Effect of ribose, xylose, aspartic acid, glutamine and nicotinic acid on ethyl (S)-4-chloro-3-hydroxybutanoate synthesis by recombinant Escherichia coli. Bioresource Technology, 118, 572–575.
Liu, L. M., Li, Y., Shi, Z. P., Du, G. C., & Chen, J. (2006). Enhancement of pyruvate productivity in Torulopsis glabrata: increase of NAD+ availability. Journal of Biotechnology, 126, 173–185.
Ma, B., Pan, S. J., Zupancic, M. L., & Cormack, B. P. (2007). Assimilation of NAD+ precursors in Candida glabrata. Molecular Microbiology, 66, 14–25.
Zhang, C. W., Xia, S. Q., & Ma, P. S. (2016). Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresource Technology, 219, 1–5.
Papadopoulou, A. A., Katsoura, M. H., Chatzikonstantinou, A., Kyriakou, E., Polydera, A. C., Tzakos, A. G., & Stamatis, H. (2013). Enzymatic hybridization of α-lipoic acid with bioactive compounds in ionic solvents. Bioresource Technology, 136, 41–48.
Xu, G. C., Ding, J. C., Han, R. Z., Dong, J. J., & Ni, Y. (2016a). Enhancing cellulose accessibility of corn Stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresource Technology, 203, 364–369.
Xu, P., Du, P. X., Zong, M. H., Li, N., & Lou, W. Y. (2016b). Combination of deep eutectic solvent and ionic liquid to improve biocatalytic reduction of 2-octanone with Acetobacter pasteurianus GIM1.158 cell. Scientific Reports, 17(6), 26158.
Xu, P., Cheng, J., Lou, W. Y., & Zong, M. H. (2015). Using deep eutectic solvents to improve the resolution of racemic 1-(4-methoxyphenyl)ethanol through Acetobacter sp. CCTCC M209061 cell-mediated asymmetric oxidation. RSC Advances, 5, 6357–6364.
Zhao, H., Baker, G. A., & Holmes, S. (2011). New eutectic ionic liquids for lipase activation and enzymatic preparation of biodiesel. Organic & Biomolecular Chemistry, 9, 1908–1916.
Gorke, J. T., Srienc, F., & Kazlauskas, R. J. (2008). Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chemical Communications, 14, 1235–1237.
Su, J. H., Xu, J. H., & Wang, Z. L. (2010). Improving enzymatic production of Ginsenoside Rh2 from Rg3 by using nonionic surfactant. Applied Biochemistry and Biotechnology, 160, 1116–1123.
Ni, Y., Zhou, J. Y., & Sun, Z. H. (2012). Production of a key chiral intermediate of Betahistine with a newly isolated Kluyveromyces sp. in an aqueous two-phase system. Process Biochemistry, 47, 1042–1048.
Tang, T. X., Liu, Y., & Wu, Z. L. (2014). Characterization of a robust anti-Prelog short-chain dehydrogenase/reductase ChKRED20 from Chryseobacterium sp. CA49. Journal of Molecular Catalysis B: Enzymatic, 105, 82–88.
Xu, H., Qin, Y., Huang, Z., & Liu, Z. (2014). Characterization and site-directed mutagenesis of an α-galactosidase from the deep-sea bacterium Bacillus megaterium. Enzyme and Microbial Technology, 56, 46–52.
He, Y. C., Zhang, D. P., Lu, Y., Tao, Z. C., Ding, Y., Wang, L. Q., & Liu, F. (2015a). Biosynthesis of ethyl (S)-4-chloro-3-hydroxybutanoate with an NADH-dependent reductase (ClCR) discovered by genome data mining using a modified colorimetric screening strategy. Bioengineered, 6, 170–174.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 21102011), Higher Education Natural Science Foundation of Jiangsu Province (No. 13KJB430025), Environmental Science Research Project of Jiangsu Province (No. 2013004), the Natural Science Foundation of Jiangsu Province (No. BK20141172), Yancheng City Industrial Science and Technology Support Project (2013), and the Teaching Reform Project of Changzhou University (No. GJY2014068).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dai, Y., Huan, B., Zhang, HS. et al. Effective Biotransformation of Ethyl 4-Chloro-3-Oxobutanoate into Ethyl (S)-4-Chloro-3-Hydroxybutanoate by Recombinant E. coli CCZU-T15 Whole Cells in [ChCl][Gly]–Water Media. Appl Biochem Biotechnol 181, 1347–1359 (2017). https://doi.org/10.1007/s12010-016-2288-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2288-0