Abstract
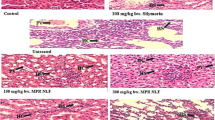
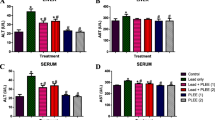
The aim of this study was to scrutinize the possible mitigating role of leaves’ Allium triquetrum L. against the toxicity of lead acetate on liver and kidney markers of Wistar rat. Lead acetate (Pb) and leaves’ aqueous extracts (L) were orally administrated for 3 weeks. Rats were divided into the control, Pb group (500 mg/kg body weight/day), positive controls L (2g, 3g, 4g/kg BW/day), along with three combined groups of the same doses (Pb-L1, Pb-L2, Pb-L3). The levels of plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total proteins (TP), albumin (ALB), urea, creatinine (Cr), and uric acid (UA), as well as the hepatic and the renal malondialdehyde (MDA), glutathione (GSH), and glutathione peroxidase (GPx), were estimated. Results exhibited a significant increase in plasma AST, ALT, ALP, urea, creatinine, uric acid, and MDA levels of the Pb group compared to the control, with the exception of TP, ALB, GSH levels, and GPx activities that were significantly diminished, though the co-administration of garlic extracts (Pb-L) revealed a significant decrease in all mentioned markers, excluding the TP, ALB, GSH, and GPx levels. Likewise, Pb caused histological injuries in the hepatic and renal tissues of rats, while the co-administration of leaves’ wild garlic has reduced such effect. Thought, the Pb-L has attenuated the Pb-induced toxicity in a dose-dependent manner. In conclusion, the aqueous extracts of A. triquetrum have the potential to alleviate Pb hepatotoxicity and nephrotoxicity through the modulation of most biomarkers in Wistar rat.





Similar content being viewed by others
Change history
11 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12011-022-03110-z
References
Kabeer A, Mailafiya MM, Danmaigoro A, Rahim EA, Bakar ZA (2019) Therapeutic potential of curcumin against lead-induced toxicity: a review. Biomed Res Ther 6:3053–3066. https://doi.org/10.15419/bmrat.v6i3.528
Gargouri M, Soussi A, Akrouti A, Magne C, El-Feki A (2019) Potential protective effects of the edible alga Arthrospira platensis against lead-induced oxidative stress, anemia, kidney injury, and histopathological changes in adult rats. Appl Physiol Nutr Metab 44:271–281. https://doi.org/10.1139/apnm-2018-0428
Mansouri O, Hamamdia Z, Abdennour C (2021) Protective effects of wheat grass on histopathology of some organs and biomarkers parameters against lead acetate toxicity in Wistar rats. J Stress Physiol Biochem 17(3):78–94
Kahalerras L, Otmani I, Abdennour C (2021) Wild garlic Allium triquetrum L. alleviates lead acetate-induced testicular injuries in rats. Biol Trace Elm Res 1-18. https://doi.org/10.1007/s12011-021-02818-8
El-Boshy ME, Refaat B, Qasem AH, Khan A, Ghaith M, Almasmoum H, Mahbub A, Almaimani RA (2019) The remedial effect of Thymus vulgaris extract against lead toxicity-induced oxidative stress, hepatorenal damage, immunosuppression, and hematological disorders in rats. Environ Sci Pollut Res 26(22):22736–22746. https://doi.org/10.1007/s11356-019-05562-8
Ezejiofor AN, Orisakwe OE (2019) Nephroprotective effect of Costusafer on lead induced kidney damage in albino rats. Int J Physiol Pathophysiol Pharmacol 11(2):36
Almatroodi SA, Anwar S, Almatroudi A, Khan AA, Alrumaihi F, AlsahliMA Rahmani, AH, (2020) Hepatoprotective effects of garlic extract against carbon tetrachloride (CCl4)-induced liver injury via modulation of antioxidant, anti-inflammatory activities and hepatocyte architecture. App Sci 10(18):6200. https://doi.org/10.3390/app10186200
El-Khishin IA, El-Fakharany YMM, Hamid OIA (2015) Role of garlic extract and silymarin compared to dimercaptosuccinic acid (DMSA) in treatment of lead induced nephropathy in adult male albino rats. Toxicol Rep 2:824–832. https://doi.org/10.1016/j.toxrep.2015.04.004
Lentini P, Zanoli L, Granata A, Signorelli SS, Castellino P, Dell’Aquila R (2017) Kidney and heavy metals-the role of environmental exposure. Mol Med Rep 15(5):3413–3419. https://doi.org/10.3892/mmr.2017.6389
Al-Megrin WA, Soliman D, Kassab RB, Metwally DM, Abdel Moneim AE, El-Khadragy MF (2020) Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front Physiol 11:64. https://doi.org/10.3389/fphys.2020.00064
Radad K, Hassanein K, Al-Shraim M, Moldzio R, Rausch WD (2014) Thymoquinone ameliorates lead-induced brain damage in Sprague Dawley rats. Exp Toxicol Pathol 66:13. https://doi.org/10.1016/j.etp.2013.07.002
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5(2):47–58. https://doi.org/10.2478/v10102-012-0009-2
Sun D, Sun C, Qiu G, Yao L, Yu J, Al Sberi H, Fouda MS, Othman MS, Lokman MS, Kassab RB, Abdel Moneim EA (2021) Allicin mitigates hepatic injury following cyclophosphamide administration via activation of Nrf2/ARE pathways and through inhibition of inflammatory and apoptotic machinery. Environ SciPollut Res 1–12https://doi.org/10.1007/s11356-021-13392-w
Patra R, Swarup D, Dwivedi S (2001) Antioxidant effects of α- tocopherol, ascorbic acid and L-methionine on lead induced oxidative stress to the liver, kidney and brain in rats. Toxicol 162:81–88. https://doi.org/10.1016/S0300-483X(01)00345-6
Ghildiyal R, Prakash V, Chaudhary VK, Gupta V, Gabrani R (2020) Phytochemicals as antiviral agents: recent updates. In Plant-derived bioactives: Production, Properties and Therapeutic Applications. Swamy MK (Ed). Springer, Singapore 12:279–295. https://doi.org/10.1007/978-981-15-1761-7_12
Al-Snafi AE (2015) The therapeutic importance of Cassia occidentalis - an overview. Indian J Pharma Sci Res 5(3):158–171
Otmani I, Abdennour C, Dridi A, Kahalerras L, Halima-Salem A (2019) Characteristics of the bitter and sweet honey from Algeria Mediterranean coast. Vet World 12(4):551–557. https://doi.org/10.14202/vetworld.2019.551-557
Anwar S, Younus H (2017) Inhibitory effect of alliin from Allium sativum on the glycation of super oxide dismutase. Int J Biol Macromol 103:182–193. https://doi.org/10.1016/j.ijbiomac.2017.05.043
El-Sebaey AM, Abdelhamid FM, Abdalla OA (2019) Protective effects of garlic extract against hematological alterations, immunosuppression, hepatic oxidative stress, and renal damage induced by cyclophosphamide in rats. Envir Sci Pollut Res 26(15):15559–15572. https://doi.org/10.1007/s11356-019-04993-7
Fihri AF, Al-Waili NS, El-Haskoury R, Bakour M, Amarti A, Ansari MJ, Lyoussi B (2016) Protective effect of Morocco carob honey against lead-induced anemia and hepato-renal toxicity. Cell Physiol Biochem 39:115–122. https://doi.org/10.1159/000445610
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. https://doi.org/10.1016/00032697(79)90738-3
Weckbecker G, Cory JG (1988) Ribonucleotide reductase activity and growth of glutathione-depleted mouse leukemia L1210 cells in vitro. Cancer Lett 40:257–264. https://doi.org/10.1016/0304-3835(88)90084-5
Flohé L, Günzler WA (1984) [12] Assays of glutathione peroxidase. Methods Enzymol 105:114–120. https://doi.org/10.1016/S0076-6879(84)05015-1 (Academic Press)
Hould R (1984) Methode de Fontana : Techniques d’histopathologie et de cytopathologie. Ed Maloine. Canada, France 19-21: 225-227
Corea G, Fattorusso E, Lanzotti V (2003) Saponins and flavonoids of Allium triquetrum. J Nat Prod 66:1405–1411. https://doi.org/10.1021/np030226q
Menacer A, Boukhatem MN, Benhelal A, Saïdi F (2017) In vitro antioxidant activity of different extracts of Algerian Allium plant (Allium triquetrum L.). Rev des Bio-Ressources 7:80–91. https://doi.org/10.12816/0045885
Menacer A, Saidi F, Benhelal A (2017) In vitro evaluation de l’activité antimicrobienne des différents extraits d’Allium triquetrum L., espèce algérienne spontané. [In vitro evaluation of the antimicrobial activity of different extracts of Allium triquetrum L., a spontaneous Algerian species]. Rev El-Wahat pour les Rech 10:152–161
Rabah S, Kouachi K, Ramos PA, Gomes AP, Almeida A, Haddadi-Guemghar H, Khodir M, Silvestre AJD, Santos SAO (2020) Unveiling the bioactivity of Allium triquetrum L. lipophilic fractions: chemical characterization and in vitro antibacterial activity against methicillin-resistant Staphylococcus aureus. Food Funct 11(6):5257–5265. https://doi.org/10.1039/D0FO00769B
Al-Brakati AY, Fouda MS, Tharwat AM, Elmahallawy EK, Kassab RB, Abdel Moneim AE (2019) The protective efficacy of soursop fruit extract against hepatic injury associated with acetaminophen exposure is mediated through antioxidant, anti-inflammatory, and antiapoptotic activities. Environ Sci Pollut Res Int 26(13):13539–13550. https://doi.org/10.1007/s11356-019-04935-3
Singh C, Prakash C, Tiwari KN, Mishra SK, Kumar V (2018) Premna integrifolia ameliorates cyclophosphamide-induced hepatotoxicity by modulation of oxidative stress and apoptosis. Biomedi Pharmacother Biomed Pharmacother 107:634–643. https://doi.org/10.1016/j.biopha.2018.08.039
Nallagangula KS, Nagaraj SK, Venkataswamy L, Chandrappa M (2017) Liver fibrosis: a compilation on the biomarker’s status and their significance during disease progression. Future Sci OA 4(1): FSO250 https://doi.org/10.4155/fsoa-2017-0083
Shin JH, Lee CW, Oh SJ, Yun J, Kang MR, Han SB, Park H, Jung JC, Chung YH, Kang JS (2014) Hepatoprotective effect of aged black garlic extract in rodents. Toxicol Res 30:49–54. https://doi.org/10.5487/TR.2014.30.1.049
Pathak S, Catanzaro R, Vasan D, Marotta F, Chabria Y, Jothimani G, Verma RS, Ramachandran M, Khuda-Bukhsh AR, Banerjee A (2018) Benefits of aged garlic extract in modulating toxicity biomarkers against p-dimethyl amino azobenzene and phenobarbital induced liver damage in Rattus norvegicus. Drug Chem Toxicol 43(5):454–467. https://doi.org/10.1080/01480545.2018.1499773
Naji KM, Al-Shaibani ES, Alhadi FA, Al-Soudi SA, D’Souza MR (2017) Hepatoprotective and antioxidant effects of single clove garlic against CCl4-induced hepatic damage in rabbits. BMC Complement Altern Med 17:1–12. https://doi.org/10.1186/s12906-017-1916-8
Aly SM, Fetaih HA, Hassanin AA, Abomughaid MM, Ismail AA (2019) Protective effects of garlic and cinnamon oils on hepatocellular carcinoma in albino rats. Anal Cell Pathol 22:9895485. https://doi.org/10.1155/2019/9895485
Navas-Acien A, Tellez-Plaza M, Guallar E, Muntner P, Silbergeld E, Jaar B, Weaver V (2009) Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am J Epidemiol 170(9):1156–64. https://doi.org/10.1093/aje/kwp248
Fadrowski JJ, Navas-Acien A, Tellez-Plaza M, Guallar E, Weaver VM, Furth SL (2010) Blood lead level and kidney function in US adolescents: The Third National Health and Nutrition Examination Survey. Arch Intern Med 170(1):75–82. https://doi.org/10.1001/archinternmed.2009.417
Lakshmi BV, Sudhakar M, Aparna M (2013) Protective potential of black grapes against lead induced oxidative stress in rats. Environ Toxicol Pharmacol 35:361–368. https://doi.org/10.1016/j.etap.2013.01.008
Shalan MG, Mostafa MS, Hassouna MM, El-Nabi SEH, El-Refaie A (2005) Amelioration of lead toxicity on rat liver with vitamin C and silymarin supplements. Toxicol 206:1–15. https://doi.org/10.1016/j.tox.2004.07.006
Dobrakowski M, Pawlas N, Kasperczyk A, Kozłowska A, Olewińska E, Machoń-Grecka A, Kasperczyk S (2016) Oxidative DNA damage and oxidative stress in lead-exposed workers. Hum Exp Toxicol 36:744–754. https://doi.org/10.1177/0960327116665674
Zhang Z, Gao X, GuoM Jiang H, Cao Y, Zhang N (2017) The protective effect of baicalin against lead-induced renal oxidative damage in mice. Biol Trace Elem Res 175:129–135. https://doi.org/10.1007/s12011-016-0731-2
Renugadevi J, Prabu SM (2010) Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp Toxicol Pathol 62:171–181. https://doi.org/10.1016/j.etp.2009.03.010
Hsu PC, Guo YL (2002) Antioxidant nutrients and lead toxicity. Toxicology 180:33–44. https://doi.org/10.1016/s0300-483x(02)00380-3
Liu CM, Liu CM, Zheng YL, Lu J, Zhang ZF, Fan SH, Wu DM, Ma JQ (2010) Quercetin protects rat liver against lead-induced oxidative stress and apoptosis. Environ Toxicol Pharmacol 29(2):158–166. https://doi.org/10.1016/j.etap.2009.12.006
Sivaprasad R, Nagaraj M, Varalakshmi P (2004) Combined efficacies of lipoic acid and 2, 3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J Nutr Biochem 15:18–23. https://doi.org/10.1016/j.jnutbio.2003.09.001
Asdaq SM, Inamdar MN (2010) Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomed 17:1016–26. https://doi.org/10.1016/j.phymed.2010.07.012
Upadhyay RK (2017) Nutritional and therapeutic potential of Allium vegetables. J Nutr Ther 6(1):18–37. https://doi.org/10.6000/1929-5634.2017.06.01.3
Abubakar K, Mailafiya MM, Chiroma SM, Danmaigoro A, Zyoud TY, Abdul Rahim E, Abu Bakar Zakaria MZ (2020) Ameliorative effect of curcumin on lead-induced hematological and hepatorenal toxicity in a rat model. J Biochem Mol Toxicol 34(6):e22483. https://doi.org/10.1002/jbt.22483
Andjelkovic M, BuhaDjordjevic A, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Spasojevic-Kalimanovska V, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019) Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16(2):274. https://doi.org/10.3390/ijerph16020274
Munday R, Munday CM (2004) Induction of phase II enzymes by aliphatic sulfides derived from garlic and onions: an overview. Methods Enzymol 382:449–456. https://doi.org/10.1016/S0076-6879(04)82024-X
Hussein JS, Oraby FS, El-Shafey N (2007) Antihepatotoxic effect of garlic and onion oils on ethanol-induced liver injury in rats. J Appl Sci Res 3(11):1527–1533
ManojKumar V, Henley AK, Nelson CJ, Indumati O, Prabhakara Rao Y, Rajanna S, Rajanna B (2016) Protective effect of Allium sativum (garlic) aqueous extract against lead-induced oxidative stress in the rat brain, liver, and kidney. Environ Sci Pollut Res 24:1544–1552. https://doi.org/10.1007/s11356-016-7923-3
Acknowledgements
The authors would like to thank the General Directorate of Scientific Research and Technological Development (DGRSDT) for supporting this project (award number 04/2016).
Funding
The authors received financial support for bench fees, but not for authorship, and/or for publication of this article.
The datasets generated during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
L Kahalerras drafted the manuscript and contributed to the analysis and interpretation of results; I Otmani participated in the experimental work; C Abdennour corrected and revised the manuscript. All authors read and gave the final approval of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
The experiment was carried out in accordance with the rules of the national guidelines of animals’ rights, and the international animal experimentation chart and animal handling of Helsinki Declaration of 2008.
Consent for Publication
Not applicable for this section.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kahalerras, L., Otmani, I. & Abdennour, C. The Allium triquetrum L. Leaves Mitigated Hepatotoxicity and Nephrotoxicity Induced by Lead Acetate in Wistar Rats. Biol Trace Elem Res 200, 4733–4743 (2022). https://doi.org/10.1007/s12011-021-03052-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-021-03052-y