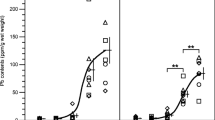
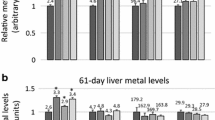
Abstract
Lead (Pb), a corrosion-resistant heavy non-ferrous metal, is one of the most common environmental neurotoxic metals. The effects of Pb on other essential metal elements are contradictory. Therefore, this in vivo study addressed the effects of sub-chronic Pb exposure on the distribution of other divalent metals, exploring the relationships between Pb levels in blood, teeth, bones, hair, and brain tissues. Thirty-two healthy male C57BL/6 mice received intragastric administration (i.g.) with 0, 12.5, 25, and 50 mg/kg Pb acetate, once a day for 8 weeks. Levels of Pb and other metal elements [including iron(Fe), zinc (Zn), magnesium (Mg), copper (Cu), and calcium(Ca)] in the whole blood, teeth, the right thighbone, hair, and brain tissues (including cortex, hippocampus, striatum, and hypothalamus) were detected with inductively coupled plasma–mass spectrometry (ICP-MS). Pb levels in all detected organs were increased after Pb-exposed for 8 weeks. The results of relationship analysis between Pb levels in the tissues and lifetime cumulative Pb exposure (LCPE) showed that Pb levels in the blood, bone, and hair could indirectly reflect the Pb accumulation in the murine brain. These measures might serve as valuable biomarkers for chronic Pb exposure reflective of the accumulation of Pb in the central nervous system (CNS). Sub-chronic Pb exposure for 8 weeks altered Ca, Cu, Fe, and Zn levels, but no effects were noted on Mg levels in any of the analyzed tissues. Pb decreased Ca in teeth, Cu in thighbone and teeth, Zn in whole blood and hair, and Fe in hair. In contrast, Pb increased Ca levels in corpus striatum and hypothalamus, Cu levels in striatum, Zn levels in teeth, and Fe levels in hippocampus, thighbone, and teeth. The Pb-induced changes in metal ratios in various tissues may serve as valuable biomarkers for chronic Pb exposure as they are closely related to the accumulations of Pb in the murine CNS. The results suggest that altered distribution of several essential metal elements may be involved in Pb-induced neurotoxicity. Additional studies should address the interaction between Pb and essential metal elements in the CNS and other organs.






Similar content being viewed by others
Change history
19 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12011-022-03195-6
References
Cheng H, Hu Y (2010) Lead (Pb) isotopic fingerprinting and its applications in lead pollution studies in China: a review. Environ Pollut 158(5):1134–1146. https://doi.org/10.1016/j.envpol.2009.12.028
Frank JJ, Poulakos AG, Tornero-Velez R, Xue J (2019) Systematic review and meta-analyses of lead (Pb) concentrations in environmental media (soil, dust, water, food, and air) reported in the United States from 1996 to 2016. Sci Total Environ 694:133489. https://doi.org/10.1016/j.scitotenv.2019.07.295
Zhou Y, Huang W, Liu X, Cao W, Wang D, Liu X, Pang Y, Wen S, Zhang X (2020) Interannual variation and exposure risk assessment of lead in brick tea in Hubei China. Sci Total Environ 745:141004. https://doi.org/10.1016/j.scitotenv.2020.141004
Kan X, Dong Y, Feng L, Zhou M, Hou H (2021) Contamination and health risk assessment of heavy metals in China’s lead-zinc mine tailings: a meta-analysis. Chemosphere 267:128909. https://doi.org/10.1016/j.chemosphere.2020.128909
Hou S, Zheng N, Tang L, Ji X, Li Y, Hua X (2019) Pollution characteristics, sources, and health risk assessment of human exposure to Cu, Zn, Cd and Pb pollution in urban street dust across China between 2009 and 2018. Environ Int 128:430–437. https://doi.org/10.1016/j.envint.2019.04.046
Taggart MA, Shore RF, Pain DJ, Peniche G, Martinez-Haro M, Mateo R, Homann J, Raab A, Feldmann J, Lawlor AJ, Potter ED, Walker LA, Braidwood DW, French AS, Parry-Jones J, Swift JA, Green RE (2020) Concentration and origin of lead (Pb) in liver and bone of Eurasian buzzards (Buteo buteo) in the United Kingdom. Environ Pollut 267:115629. https://doi.org/10.1016/j.envpol.2020.115629
Shih RA, Glass TA, Bandeen-Roche K, Carlson MC, Bolla KI, Todd AC, Schwartz BS (2006) Environmental lead exposure and cognitive function in community-dwelling older adults. Neurology 67(9):1556–1562. https://doi.org/10.1212/01.wnl.0000239836.26142.c5
Galiciolli MEA, Lima LS, da Costa NS, de Andrade DP, Irioda AC, Oliveira CS (2022) IQ alteration induced by lead in developed and underdeveloped/developing countries: a systematic review and a meta-analysis. Environ Pollut 292(Pt A):118316. https://doi.org/10.1016/j.envpol.2021.118316
Pedroso TF, Oliveira CS, Fonseca MM, Oliveira VA, Pereira ME (2017) Effects of zinc and N-acetylcysteine in damage caused by lead exposure in young rats. Biol Trace Elem Res 180(2):275–284. https://doi.org/10.1007/s12011-017-1009-z
Vizuete J, Perez-Lopez M, Miguez-Santiyan MP, Hernandez-Moreno D (2019) Mercury (Hg), lead (Pb), cadmium (Cd), selenium (Se), and arsenic (As) in liver, kidney, and feathers of gulls: a review. Rev Environ Contam Toxicol 247:85–146. https://doi.org/10.1007/398_2018_16
Mohammadyan M, Moosazadeh M, Borji A, Khanjani N, Rahimi Moghadam S (2019) Exposure to lead and its effect on sleep quality and digestive problems in soldering workers. Environ Monit Assess 191(3):184. https://doi.org/10.1007/s10661-019-7298-2
Brown L, Lynch M, Belova A, Klein R, Chiger A (2020) Developing a health impact model for adult lead exposure and cardiovascular disease mortality. Environ Health Perspect 128(9):97005. https://doi.org/10.1289/EHP6552
Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ (2007) Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspect 115(3):472–482. https://doi.org/10.1289/ehp.9785
Boskabady MH, Tabatabai SA, Farkhondeh T (2016) Inhaled lead affects lung pathology and inflammation in sensitized and control guinea pigs. Environ Toxicol 31(4):452–460. https://doi.org/10.1002/tox.22058
Jurdziak M, Gac P, Martynowicz H, Poreba R (2015) Function of respiratory system evaluated using selected spirometry parameters in persons occupationally exposed to lead without evident health problems. Environ Toxicol Pharmacol 39(3):1034–1040. https://doi.org/10.1016/j.etap.2015.03.009
Jacob B, Ritz B, Heinrich J, Hoelscher B, Wichmann HE (2000) The effect of low-level blood lead on hematologic parameters in children. Environ Res 82(2):150–159. https://doi.org/10.1006/enrs.1999.4011
Chen Y, Xu X, Zeng Z, Lin X, Qin Q, Huo X (2019) Blood lead and cadmium levels associated with hematological and hepatic functions in patients from an e-waste-polluted area. Chemosphere 220:531–538. https://doi.org/10.1016/j.chemosphere.2018.12.129
Fenga C, Gangemi S, Di Salvatore V, Falzone L, Libra M (2017) Immunological effects of occupational exposure to lead (review). Mol Med Rep 15(5):3355–3360. https://doi.org/10.3892/mmr.2017.6381
Gibson JM, Fisher M, Clonch A, MacDonald JM, Cook PJ (2020) Children drinking private well water have higher blood lead than those with city water. Proc Natl Acad Sci U S A 117(29):16898–16907. https://doi.org/10.1073/pnas.2002729117
Liu F, Wang Z, Wei Y, Liu R, Jiang C, Gong C, Liu Y, Yan B (2021) The leading role of adsorbed lead in PM2.5-induced hippocampal neuronal apoptosis and synaptic damage. J Hazard Mater 416:125867. https://doi.org/10.1016/j.jhazmat.2021.125867
Liu CM, Zheng GH, Cheng C, Sun JM (2013) Quercetin protects mouse brain against lead-induced neurotoxicity. J Agric Food Chem 61(31):7630–7635. https://doi.org/10.1021/jf303387d
Lidsky TI, Schneider JS (2003) Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain 126(Pt 1):5–19. https://doi.org/10.1093/brain/awg014
Verstraeten SV, Aimo L, Oteiza PI (2008) Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol 82(11):789–802. https://doi.org/10.1007/s00204-008-0345-3
Cheng, H., B. Yang, T. Ke, S. Li, X. Yang, M. Aschner, and P. Chen (2021) Mechanisms of metal-induced mitochondrial dysfunction in neurological disorders. Toxics. 9(6) https://doi.org/10.3390/toxics9060142.
Park SJ, Lee JH, Woo SJ, Kang SW, Park KH, S Epidemiologic Survey Committee of Korean Ophthalmologic (2015) Five heavy metallic elements and age-related macular degeneration: Korean National Health and Nutrition Examination Survey, 2008-2011. Ophthalmology 122(1):129–37. https://doi.org/10.1016/j.ophtha.2014.07.039
Kawahara, M., K.I. Tanaka, and M. Kato-Negishi (2018) Zinc, carnosine, and neurodegenerative diseases. Nutrients. 10(2) https://doi.org/10.3390/nu10020147.
Mezzaroba L, Alfieri DF, Colado Simao AN, Vissoci Reiche EM (2019) The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 74:230–241. https://doi.org/10.1016/j.neuro.2019.07.007
Benin AL, Sargent JD, Dalton M, Roda S (1999) High concentrations of heavy metals in neighborhoods near ore smelters in northern Mexico. Environ Health Perspect 107(4):279–284. https://doi.org/10.1289/ehp.99107279
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6(6):449–462. https://doi.org/10.1038/nrn1671
Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW (2021) Iron deficiency. Lancet 397(10270):233–248. https://doi.org/10.1016/S0140-6736(20)32594-0
Simunkova M, Alwasel SH, Alhazza IM, Jomova K, Kollar V, Rusko M, Valko M (2019) Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch Toxicol 93(9):2491–2513. https://doi.org/10.1007/s00204-019-02538-y
Yu Y, Yu L, Zhou X, Qiao N, Qu D, Tian F, Zhao J, Zhang H, Zhai Q, Chen W (2020) Effects of acute oral lead exposure on the levels of essential elements of mice: a metallomics and dose-dependent study. J Trace Elem Med Biol 62:126624. https://doi.org/10.1016/j.jtemb.2020.126624
Miller GD, Massaro TF, Massaro EJ (1990) Interactions between lead and essential elements: a review. Neurotoxicology 11(1):99–119
Liu ZH, Shang J, Yan L, Wei T, Xiang L, Wang HL, Cheng J, Xiao G (2020) Oxidative stress caused by lead (Pb) induces iron deficiency in Drosophila melanogaster. Chemosphere 243:125428. https://doi.org/10.1016/j.chemosphere.2019.125428
Li Y, Li M, Lv Q, Chen G, Chen J, Li S, Mo Y, Ou S, Yuan Z, Lu G, Jiang Y (2015) Relationship of lead and essential elements in whole blood from school-age children in Nanning China. J Trace Elem Med Biol 32:107–111. https://doi.org/10.1016/j.jtemb.2015.06.007
Bressler JP, Olivi L, Cheong JH, Kim Y, Bannona D (2004) Divalent metal transporter 1 in lead and cadmium transport. Ann N Y Acad Sci 1012:142–152. https://doi.org/10.1196/annals.1306.011
Shah F, Kazi TG, Afridi HI, Baig JA, Khan S, Kolachi NF, Wadhwa SK, Shah AQ (2010) Environmental exposure of lead and iron deficit anemia in children age ranged 1–5 years: a cross sectional study. Sci Total Environ 408(22):5325–5330. https://doi.org/10.1016/j.scitotenv.2010.07.091
Aimo L, Oteiza PI (2006) Zinc deficiency increases the susceptibility of human neuroblastoma cells to lead-induced activator protein-1 activation. Toxicol Sci 91(1):184–191. https://doi.org/10.1093/toxsci/kfj137
Lin F, Fan G-q, Feng C, Feng F-y, Yan J, Wang H-h, Huang Y, Xiao Y-m, Kuang B-h, Li Y-s (2010) Effects of lead exposure through drinking water on content of iron,zinc,manganese and copper in different encephalic regions of rats. Journal of Environment and Health 27(07):578–580. https://doi.org/10.16241/j.cnki.1001-5914.2010.07.029
Cobbina SJ, Chen Y, Zhou Z, Wu X, Feng W, Wang W, Li Q, Zhao T, Mao G, Wu X, Yang L (2015) Interaction of four low dose toxic metals with essential metals in brain, liver and kidneys of mice on sub-chronic exposure. Environ Toxicol Pharmacol 39(1):280–291. https://doi.org/10.1016/j.etap.2014.11.030
Jiang X, Xing X, Zhang Y, Zhang C, Wu Y, Chen Y, Meng R, Jia H, Cheng Y, Zhang Y, Su J (2021) Lead exposure activates the Nrf2/Keap1 pathway, aggravates oxidative stress, and induces reproductive damage in female mice. Ecotoxicol Environ Saf 207:111231. https://doi.org/10.1016/j.ecoenv.2020.111231
Yu C, Zhang J, Li X, Liu J, Niu Y (2021) Astragaloside IV-induced Nrf2 nuclear translocation ameliorates lead-related cognitive impairments in mice. Biochim Biophys Acta Mol Cell Res 1868(1):118853. https://doi.org/10.1016/j.bbamcr.2020.118853
Yang QW, Shu WS, Qiu JW, Wang HB, Lan CY (2004) Lead in paddy soils and rice plants and its potential health risk around Lechang lead/zinc mine, Guangdong China. Environ Int 30(7):883–889. https://doi.org/10.1016/j.envint.2004.02.002
Mielke HW, Laidlaw MA, Gonzales CR (2011) Estimation of leaded (Pb) gasoline’s continuing material and health impacts on 90 US urbanized areas. Environ Int 37(1):248–257. https://doi.org/10.1016/j.envint.2010.08.006
Shi T, Ma J, Zhang Y, Liu C, Hu Y, Gong Y, Wu X, Ju T, Hou H, Zhao L (2019) Status of lead accumulation in agricultural soils across China (1979–2016). Environ Int 129:35–41. https://doi.org/10.1016/j.envint.2019.05.025
Singh P, Mitra P, Goyal T, Sharma S, Purohit P, Sharma P (2021) Levels of lead, aluminum, and zinc in occupationally exposed workers of North-Western India. J Basic Clin Physiol Pharmacol. https://doi.org/10.1515/jbcpp-2020-0220
Tong S, von Schirnding YE, Prapamontol T (2000) Environmental lead exposure: a public health problem of global dimensions. Bull World Health Organ 78(9):1068–1077
Mitra P, Sharma S, Purohit P, Sharma P (2017) Clinical and molecular aspects of lead toxicity: An update. Crit Rev Clin Lab Sci 54(7–8):506–528. https://doi.org/10.1080/10408363.2017.1408562
Mielke HW, Gonzales CR, Powell ET, Mielke PW (2013) Environmental and health disparities in residential communities of New Orleans: the need for soil lead intervention to advance primary prevention. Environ Int 51:73–81. https://doi.org/10.1016/j.envint.2012.10.013
Baranowska-Bosiacka I, Kosinska I, Jamiol D, Gutowska I, Prokopowicz A, Rebacz-Maron E, Goschorska M, Olszowski T, Chlubek D (2016) Environmental lead (Pb) exposure versus fatty acid content in blood and milk of the mother and in the blood of newborn children. Biol Trace Elem Res 170(2):279–287. https://doi.org/10.1007/s12011-015-0482-5
Database, P.P.C., Lead, Access 26 March 2015.
Ge X, Liu Z, Hou Q, Huang L, Zhou Y, Li D, Huang S, Luo X, Lv Y, Li L, Cheng H, Chen X, Zan G, Tan Y, Liu C, Zou Y, Yang X (2020) Plasma metals and serum bilirubin levels in workers from manganese-exposed workers healthy cohort (MEWHC). Environ Pollut 258:113683. https://doi.org/10.1016/j.envpol.2019.113683
Ge X, Wang F, Zhong Y, Lv Y, Jiang C, Zhou Y, Li D, Xia B, Su C, Cheng H, Ma Y, Xiong F, Shen Y, Zou Y, Yang X (2018) Manganese in blood cells as an exposure biomarker in manganese-exposed workers healthy cohort. J Trace Elem Med Biol 45:41–47. https://doi.org/10.1016/j.jtemb.2017.09.016
Liu KS, Hao JH, Zeng Y, Dai FC, Gu PQ (2013) Neurotoxicity and biomarkers of lead exposure: a review. Chin Med Sci J 28(3):178–188. https://doi.org/10.1016/s1001-9294(13)60045-0
Kalahasthi R, Barman T, Bagepally BS (2020) Assessment of bone turnover biomarkers in lead-battery workers with long-term exposure to lead. Int J Occup Environ Med 11(3):140–147. https://doi.org/10.34172/ijoem.2020.1951
Sakai T (2000) Biomarkers of lead exposure. Ind Health 38(2):127–142. https://doi.org/10.2486/indhealth.38.127
Levin-Schwartz Y, Gennings C, Claus Henn B, Coull BA, Placidi D, Lucchini R, Smith DR, Wright RO (2020) Multi-media biomarkers: integrating information to improve lead exposure assessment. Environ Res 183:109148. https://doi.org/10.1016/j.envres.2020.109148
Olympio K, Ferreira A, Rodrigues M, Luz MS, Albuquerque L, Barbosa J, Cardoso M, Oliveira PV, Buzalaf M (2020) Are fingernail lead levels a reliable biomarker of lead internal dose? J Trace Elem Med Biol 62:126576. https://doi.org/10.1016/j.jtemb.2020.126576
Fortin MC, Cory-Slechta DA, Ohman-Strickland P, Nwankwo C, Yanger TS, Todd AC, Moynihan J, Walton J, Brooks A, Fiedler N (2012) Increased lead biomarker levels are associated with changes in hormonal response to stress in occupationally exposed male participants. Environ Health Perspect 120(2):278–283. https://doi.org/10.1289/ehp.1103873
Tian L, Zheng G, Sommar JN, Liang Y, Lundh T, Broberg K, Lei L, Guo W, Li Y, Tan M, Skerfving S, Jin T, Bergdahl IA (2013) Lead concentration in plasma as a biomarker of exposure and risk, and modification of toxicity by delta-aminolevulinic acid dehydratase gene polymorphism. Toxicol Lett 221(2):102–109. https://doi.org/10.1016/j.toxlet.2013.06.214
Klotz, K. and T. Goen (2017) Human biomonitoring of lead exposure. Met Ions Life Sci. 17 https://doi.org/10.1515/9783110434330-006.
Mohammadyan M, Moosazadeh M, Borji A, Khanjani N, Rahimi Moghadam S (2019) Investigation of occupational exposure to lead and its relation with blood lead levels in electrical solderers. Environ Monit Assess 191(3):126. https://doi.org/10.1007/s10661-019-7258-x
Dantzer J, Ryan P, Yolton K, Parsons PJ, Palmer CD, Cecil K, Unrine JM (2020) A comparison of blood and toenails as biomarkers of children’s exposure to lead and their correlation with cognitive function. Sci Total Environ 700:134519. https://doi.org/10.1016/j.scitotenv.2019.134519
Bjorklund G, Dadar M, Peana M, Rahaman MS, Aaseth J (2020) Interactions between iron and manganese in neurotoxicity. Arch Toxicol 94(3):725–734. https://doi.org/10.1007/s00204-020-02652-2
Fiore M, Barone R, Copat C, Grasso A, Cristaldi A, Rizzo R, Ferrante M (2020) Metal and essential element levels in hair and association with autism severity. J Trace Elem Med Biol 57:126409. https://doi.org/10.1016/j.jtemb.2019.126409
Kim J, Wessling-Resnick M (2014) Iron and mechanisms of emotional behavior. J Nutr Biochem 25(11):1101–1107. https://doi.org/10.1016/j.jnutbio.2014.07.003
Lane DJR, Ayton S, Bush AI (2018) Iron and Alzheimer’s disease: an update on emerging mechanisms. J Alzheimers Dis 64(s1):S379–S395. https://doi.org/10.3233/JAD-179944
Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139(Suppl 1):179–197. https://doi.org/10.1111/jnc.13425
Zhu G, Fan G, Feng C, Li Y, Chen Y, Zhou F, Du G, Jiao H, Liu Z, Xiao X, Lin F, Yan J (2013) The effect of lead exposure on brain iron homeostasis and the expression of DMT1/FP1 in the brain in developing and aged rats. Toxicol Lett 216(2–3):108–123. https://doi.org/10.1016/j.toxlet.2012.11.024
Kordas K (2010) Iron, lead, and children’s behavior and cognition. Annu Rev Nutr 30:123–148. https://doi.org/10.1146/annurev.nutr.012809.104758
Zhou F, Chen Y, Fan G, Feng C, Du G, Zhu G, Li Y, Jiao H, Guan L, Wang Z (2014) Lead-induced iron overload and attenuated effects of ferroportin 1 overexpression in PC12 cells. Toxicol In Vitro 28(8):1339–1348. https://doi.org/10.1016/j.tiv.2014.07.005
Rondo PH, Conde A, Souza MC, Sakuma A (2011) Iron deficiency anaemia and blood lead concentrations in Brazilian children. Trans R Soc Trop Med Hyg 105(9):525–530. https://doi.org/10.1016/j.trstmh.2011.05.012
Kaul B (1999) Lead exposure and iron deficiency among Jammu and New Delhi children. Indian J Pediatr 66(1):27–35. https://doi.org/10.1007/BF02752347
Rondo PH, Carvalho Mde F, Souza MC, Moraes F (2006) Lead, hemoglobin, zinc protoporphyrin and ferritin concentrations in children. Rev Saude Publica 40(1):71–76. https://doi.org/10.1590/s0034-89102006000100012
Volpe SL (2013) Magnesium in disease prevention and overall health. Adv Nutr 4(3):378S-S383. https://doi.org/10.3945/an.112.003483
Veronese N, Zurlo A, Solmi M, Luchini C, Trevisan C, Bano G, Manzato E, Sergi G, Rylander R (2016) Magnesium status in Alzheimer’s disease: a systematic review. Am J Alzheimers Dis Other Demen 31(3):208–213. https://doi.org/10.1177/1533317515602674
Wyparlo-Wszelaki M, Wasik M, Machon-Grecka A, Kasperczyk A, Bellanti F, Kasperczyk S, Dobrakowski M (2021) Blood magnesium level and selected oxidative stress indices in lead-exposed workers. Biol Trace Elem Res 199(2):465–472. https://doi.org/10.1007/s12011-020-02168-x
Anetor JI, Ajose OA, Adebiyi JA, Akingbola TS, Iyanda AA, Ebesunu MO, Babalola OO, Aadeniyi FA (2007) Decreased thiamine and magnesium levels in the potentiation of the neurotoxicity of lead in occupational lead exposure. Biol Trace Elem Res 116(1):43–51. https://doi.org/10.1007/BF02685917
Todorovic T, Vujanovic D, Dozic I, Petkovic-Curcin A (2008) Calcium and magnesium content in hard tissues of rats under condition of subchronic lead intoxication. Magnes Res 21(1):43–50
Lakshmi Priya MD, Geetha A (2011) Level of trace elements (copper, zinc, magnesium and selenium) and toxic elements (lead and mercury) in the hair and nail of children with autism. Biol Trace Elem Res 142(2):148–158. https://doi.org/10.1007/s12011-010-8766-2
Wu Y, Yang X, Ge J, Zhang J (2011) Blood lead level and its relationship to certain essential elements in the children aged 0 to 14 years from Beijing China. Sci Total Environ 409(16):3016–3020. https://doi.org/10.1016/j.scitotenv.2011.04.050
Dlugaszek M, Skrzeczanowski W (2017) Relationships between element contents in Polish children’s and adolescents’ hair. Biol Trace Elem Res 180(1):6–14. https://doi.org/10.1007/s12011-017-0987-1
Ye J, Du C, Wang L, Li Z, Huang S, Wang H, He L, Bi Y, Wang C (2015) Relationship of blood levels of Pb with Cu, Zn, Ca, Mg, Fe, and Hb in children aged 0 approximately 6 years from Wuhan China. Biol Trace Elem Res 164(1):18–24. https://doi.org/10.1007/s12011-014-0200-8
Kang-Sheng L, Xiao-Dong M, Juan S, Chun-Fan D, Pingqing G (2015) Towards bio monitoring of toxic (lead) and essential elements in whole blood from 1- to 72-month old children: a cross-sectional study. Afr Health Sci 15(2):634–640. https://doi.org/10.4314/ahs.v15i2.42
Miller RJ (1988) Calcium signalling in neurons. Trends Neurosci 11(10):415–419. https://doi.org/10.1016/0166-2236(88)90191-9
Nicotera P, Bellomo G, Orrenius S (1992) Calcium-mediated mechanisms in chemically induced cell death. Annu Rev Pharmacol Toxicol 32:449–470. https://doi.org/10.1146/annurev.pa.32.040192.002313
Dudev T, Grauffel C, Lim C (2018) How Pb(2+) binds and modulates properties of Ca(2+)-signaling proteins. Inorg Chem 57(23):14798–14809. https://doi.org/10.1021/acs.inorgchem.8b02548
Marchioro M, Swanson KL, Aracava Y, Albuquerque EX (1996) Glycine and calcium-dependent effects of lead on N-methyl-D-aspartate receptor function in rat hippocampal neurons. J Pharmacol Exp Ther 279(1):143–153
Ettinger AS, Lamadrid-Figueroa H, Tellez-Rojo MM, Mercado-Garcia A, Peterson KE, Schwartz J, Hu H, Hernandez-Avila M (2009) Effect of calcium supplementation on blood lead levels in pregnancy: a randomized placebo-controlled trial. Environ Health Perspect 117(1):26–31. https://doi.org/10.1289/ehp.11868
Ross EA, Szabo NJ, Tebbett IR (2000) Lead content of calcium supplements. JAMA 284(11):1425–1429. https://doi.org/10.1001/jama.284.11.1425
Oteiza PI, Mackenzie GG (2005) Zinc, oxidant-triggered cell signaling, and human health. Mol Aspects Med 26(4–5):245–255. https://doi.org/10.1016/j.mam.2005.07.012
Song MK, Adham NF, Costea NV (1984) Effect of different levels of dietary zinc on longevity of BALB/c mice inoculated with plasmacytoma MOPC 104E. J Natl Cancer Inst 72(3):647–652
Uriu-Adams JY, Keen CL (2005) Copper, oxidative stress, and human health. Mol Aspects Med 26(4–5):268–298. https://doi.org/10.1016/j.mam.2005.07.015
Feng C, Liu Y, Yuan Y, Cui W, Zheng F, Ma Y, Piao M (2016) Isoflurane anesthesia exacerbates learning and memory impairment in zinc-deficient APP/PS1 transgenic mice. Neuropharmacology 111:119–129. https://doi.org/10.1016/j.neuropharm.2016.08.035
Kumar V, Kumar A, Singh K, Avasthi K, Kim JJ (2021) Neurobiology of zinc and its role in neurogenesis. Eur J Nutr 60(1):55–64. https://doi.org/10.1007/s00394-020-02454-3
Montes S, Rivera-Mancia S, Diaz-Ruiz A, Tristan-Lopez L, Rios C (2014) Copper and copper proteins in Parkinson’s disease. Oxid Med Cell Longev 2014:147251. https://doi.org/10.1155/2014/147251
Ejaz, H.W., W. Wang, and M. Lang (2020) Copper toxicity links to pathogenesis of Alzheimer's disease and therapeutics approaches. Int J Mol Sci. 21(20) https://doi.org/10.3390/ijms21207660.
Esmieu C, Guettas D, Conte-Daban A, Sabater L, Faller P, Hureau C (2019) Copper-targeting approaches in Alzheimer’s disease: how to improve the fallouts obtained from in vitro studies. Inorg Chem 58(20):13509–13527. https://doi.org/10.1021/acs.inorgchem.9b00995
Zhao H, Yang H, Yan L, Jiang S, Xue L, Zhao H, Guan W, Pang S, Zhang Y (2014) Effects of lead exposure on copper and copper transporters in choroid plexus of rats]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 32(11):819–822
Sierra EM, Rowles TK, Martin J, Bratton GR, Womac C, Tiffany-Castiglioni E (1989) Low level lead neurotoxicity in a pregnant guinea pigs model: neuroglial enzyme activities and brain trace metal concentrations. Toxicology 59(1):81–96. https://doi.org/10.1016/0300-483x(89)90158-3
Qian Y, Mikeska G, Harris ED, Bratton GR, Tiffany-Castiglioni E (1999) Effect of lead exposure and accumulation on copper homeostasis in cultured C6 rat glioma cells. Toxicol Appl Pharmacol 158(1):41–49. https://doi.org/10.1006/taap.1999.8657
Tiffany-Castiglioni E, Garcia DM, Wu JN, Zmudzki J, Bratton GR (1988) Effects of lead on viability and intracellular metal content of C6 rat glioma cells. J Toxicol Environ Health 23(2):267–279. https://doi.org/10.1080/15287398809531112
Zhang J, Li X, Shen L, Khan NU, Zhang X, Chen L, Zhao H, Luo P (2021) Trace elements in children with autism spectrum disorder: a meta-analysis based on case-control studies. J Trace Elem Med Biol 67:126782. https://doi.org/10.1016/j.jtemb.2021.126782
Funding
This study was supported by grants from the National Natural Science Foundation of China (NSFC81803281), Guangxi Natural Science Foundation (2018GXNSFBA050060), and Guangxi Natural Science Found for Innovation Research Team (2019GXNSFGA245002).
Author information
Authors and Affiliations
Contributions
SJL made substantial contributions to the research design. SJL, CY, and XY established the animal model and analyzed the samples. RKW and SYO performed a statistical analysis of the data. MA and YMJ directed the design of the research. SJL and CCY drafted the manuscript, and MA revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, S., Yang, C., Yi, X. et al. Effects of Sub-chronic Lead Exposure on Essential Element Levels in Mice. Biol Trace Elem Res 201, 282–293 (2023). https://doi.org/10.1007/s12011-022-03137-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03137-2