Abstract
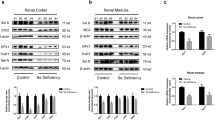
The effects of short-term dietary selenium deficiency on the liver and protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway were evaluated. Fourteen growing rats were randomly divided into control and selenium deficiency groups and fed standard and selenium-deficient diets for 4 weeks, respectively. The serum and liver selenium concentrations were measured to evaluate the construction of animal models with selenium deficiency. Liver tissues were analyzed by transmission electron microscope, hematoxylin–eosin staining, and Masson staining to observe the ultrastructural changes, pathological changes, and severity of liver fibrosis, respectively. Besides, immunohistochemical staining (IHC) was used to analyze the effects of selenium deficiency on the expression of key proteins in the Akt/mTOR signaling pathway. The results showed that selenium concentrations in the serum and liver tissue were significantly lower in the selenium deficiency group than in the control group, and the selenium deficiency intervention could affect the morphology and structure of hepatocytes and mitochondria. Meanwhile, the liver tissue showed structural damage and fibrotic changes in the selenium deficiency group. The IHC results showed the positive staining rates of Akt, phosphorylation-modified protein kinase B (p-Akt), mTOR, and phosphorylation-modified mammalian target of the rapamycin (p-mTOR) in the liver of the selenium deficiency group which were significantly lower than that of the control group. In conclusion, short-term selenium deficiency dietary intervention could lead to liver fibrosis by inhibiting the Akt/mTOR signaling pathway.






Similar content being viewed by others
References
Rayman MP (2012) Selenium and human health. Lancet 379(9822):1256–1268
Wang N, Tan HY, Li S, Xu Y, Guo W, Feng Y (2017) Supplementation of micronutrient selenium in metabolic diseases: its role as an antioxidant. Oxid Med Cell Longev 2017:7478523
Rayman MP (2000) The importance of selenium to human health. Lancet 356(9225):233–241
Avery JC, Hoffmann PR (2018) Selenium, selenoproteins, and immunity. Nutrients 10(9):1203
Qiao LC, Guo ZW, Liu HB, Liu JX, Lin X, Deng H, Liu X, Zhao Y, Xiao X, Lei J, Han J (2022) Protective effect of mitophagy regulated by mTOR signaling pathway in liver fibrosis associated with selenium. Nutrients 14(12):2410
Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1):25–54
Bulteau AL, Chavatte L (2015) Update on selenoprotein biosynthesis. Antioxid Redox Signal 23(10):775–794
Tan LC, Nancharaiah YV, van Hullebusch ED, Lens PNL (2016) Selenium: environmental significance, pollution, and biological treatment technologies. Biotechnol Adv 34(5):886–907
Ha HY, Alfulaij N, Berry MJ, Seale LA (2019) From selenium absorption to selenoprotein degradation. Biol Trace Elem Res 192(1):26–37
Shang N, Wang X, Shu Q, Wang H, Zhao L (2019) The functions of selenium and selenoproteins relating to the liver diseases. J Nanosci Nanotechnol 19(4):1875–1888
Burk RF, Hill KE (2015) Regulation of selenium metabolism and transport. Annu Rev Nutr 7(35):109–134
Lei XG, Evenson JK, Thompson KM, Sunde RA (1995) Glutathione peroxidase and phospholipid hy-droperoxide glutathione peroxidase are differentially regulated in rats by dietary selenium. J Nutr 125:1438–1446
Hill KE, Wu S, Motley AK, Stevenson TD, Winfrey VP, Capecchi MR, Atkins JF, Burk RF (2012) Production of selenoprotein P (Sepp1) by hepatocytes is central to selenium homeostasis. J Biol Chem 287:40414–40424
Han J, Liang H, Yi J, Tan W, He S, Wang S, Li F, Wu X, Ma J, Shi X, Guo X, Bai C (2017) Long-term selenium-deficient diet induces liver damage by altering hepatocyte ultrastructure and MMP1/3 and TIMP1/3 expression in growing rats. Biol Trace Elem Res 175(2):396–404
Sullivan JF, Blotcky AJ, Jetton MM, Hahn HK, Burch RE (1979) Serum levels of selenium, calcium, copper magnesium, manganese and zinc in various human diseases. J Nutr 109(8):1432–1437
Aaseth J, Alexander J, Thomassen Y, Blomhoff JP, Skrede S (1982) Serum selenium levels in liver diseases. Clin Biochem 15(6):281–283
Burk RF, Early DS, Hill KE, Palmer IS, Boeglin ME (1998) Plasma selenium in patients with cirrhosis. Hepatology 27(3):794–798
George J (2018) Determination of selenium during pathogenesis of hepatic fibrosis employing hydride generation and inductively coupled plasma mass spectrometry. Biol Chem 399(5):499–509
Petrovski BÉ, Pataki V, Jenei T, Adány R, Vokó Z (2012) Selenium levels in men with liver disease in Hungary. J Trace Elem Med Biol 26(1):31–35
Prystupa A, Kiciński P, Luchowska-Kocot D, Błażewicz A, Niedziałek J, Mizerski G, Jojczuk M, Ochal A, Sak JJ, Załuska W (2017) Association between serum selenium concentrations and levels of proinflammatory and profibrotic cytokines-interleukin-6 and growth differentiation factor-15, in patients with alcoholic liver cirrhosis. Int J Environ Res Public Health 14(4):437
Reja M, Makar M, Visaria A, Marino D, Rustgi V (2020) Increased serum selenium levels are associated with reduced risk of advanced liver fibrosis and all-cause mortality in NAFLD patients: National Health and Nutrition Examination Survey (NHANES) III. Ann Hepatol 19(6):635–640
Li J, Deng X, Wang S, Jiang Q, Xu K (2021) Resolvin D1 attenuates CCl4 induced liver fibrosis by inhibiting autophagy-mediated HSC activation via AKT/mTOR pathway. Front Pharmacol 12:792414
Jung K, Kim M, So J, Lee SH, Ko S, Shin D (2021) Farnesoid X receptor activation impairs liver progenitor cell-mediated liver regeneration via the PTEN-PI3K-AKT-mTOR axis in zebrafish. Hepatology 74(1):397–410
Zhang M, Xue Y, Zheng B, Li L, Chu X, Zhao Y, Wu Y, Zhang J, Han X, Wu Z, Chu L (2021) Liquiritigenin protects against arsenic trioxide-induced liver injury by inhibiting oxidative stress and enhancing mTOR-mediated autophagy. Biomed Pharmacother 143:112167
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22:696–699
Zhang L, Du YF, Chen YZ, Xu JY, Wang B, Qin LQ (2020) New progress of selenium and hepatic injury. Modern Preventive Medicine 47(22):4218–4220
Kvícala J (1999) Selenium and the organism. Casopís Lékar Ceskych 138(4):99–106
Schomburg L, Hughes DJ (2017) The missing link? The potential role of selenium in the development of liver cancer and significance for the general population. Expert Rev Gastroenterol Hepatol 11(8):707–709
Zhang M, Song G, Minuk GY (1996) Effects of hepatic stimulator substance, herbal medicine, selenium/vitamin E, and ciprofloxacin on cirrhosis in the rat. Gastroenterology 110(4):1150–1155
Zhao LL, Chen J, Yan LJ, Pei JR, Gao L, Li XZ, Liu MF, Li SC, Zhou LW, Liu ZQ, Wang T (2012) Effect of selenium, protein and vitamin E deficiency on mRNA expression of rat cardiac selenoprotein. Chin J Endem 31(3):279–282
Pan XQ, Wei J, Wang R, Zhu YH, Zhang M, Lin L, Dan H, Yan R, He L (2013) Effects of selenium deficiency on cardiac function and mitochondrial ultrastructure of rat myocardium. Chin J Endem 32(4):378–383
Han FC, Yuan SF (1992) Changes of activities of mitochondrial membrane binding enzymes in rats fed with low selenium diet. J Xi’an Jiaotong Univ (Medical Sciences) 13(4):316–320
Saks VA, Chernousova GB, Voronkov II, Smirnov VN, Chazov EI (1974) Study of energy transport mechanism in myocardial cells. Circ Res 35(Suppl 3):138–149
Barnett R (2018) Liver cirrhosis. Lancet 392(10144):275
Aydın MM, Akçalı KC (2018) Liver fibrosis. Turk J Gastroenterol 29(1):14–21
Schuppan D, Afdhal NH (2008) Liver cirrhosis. Lancet 371(9615):838–851
Liu L, Geng X, Cai Y, Copple B, Yoshinaga M, Shen J, Nebert DW, Wang H, Liu Z (2018) Hepatic ZIP8 deficiency is associated with disrupted selenium homeostasis, liver pathology, and tumor formation. Am J Physiol Gastrointest Liver Physiol 315(4):G569–G579
Wang R, Song F, Li S, Wu B, Gu Y, Yuan Y (2019) Salvianolic acid A attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways. Drug Des Devel Ther 13:1889–1900
Shen M, Guo M, Wang Z, Li Y, Kong D, Shao J, Tan S, Chen A, Zhang F, Zhang Z, Zheng S (2020) ROS-dependent inhibition of the PI3K/Akt/mTOR signaling is required for Oroxylin A to exert anti-inflammatory activity in liver fibrosis. Int Immunopharmacol 85:106637
Wu CH, Shen M, Li L, Wei J (2015) Association between PI3K/Akt/mTOR/p70S6K signaling pathway and hepatic fibrosis. J Clin Hepatol 31(11):1928–1932
Huang C, Li J, Ma TT (2011) PI3K/Akt signaling pathway and liver fibrosis. Chin Pharmaco Bull 27(8):1037–1041
Acknowledgements
We thank Jian Lei and Huan Deng for their participation in the experiments of this study.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81872567).
Author information
Authors and Affiliations
Contributions
Study design: Jing Han and Lichun Qiao. Animal experiments: Lichun Qiao, Xue Lin, Yan Zhao, and Haobiao Liu. Data collection: Ziwei Guo, Qingfeng Wang, and Mei You. Statistical analysis: Zhihao Yang and Qian Yuan. Figure preparation: Xue Lin, Jiaxin Liu, and Wenming Bian. Manuscript preparation: Lichun Qiao and Jing Han. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study protocol was approved by the Medical Animal Research Ethics Committee of Xi’an Jiaotong University (protocol code 2018.263 and 6 March 2018 of approval).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiao, L., Lin, X., Zhao, Y. et al. Short-term Dietary Selenium Deficiency Induced Liver Fibrosis by Inhibiting the Akt/mTOR Signaling Pathway in Rats. Biol Trace Elem Res 201, 3825–3833 (2023). https://doi.org/10.1007/s12011-022-03453-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03453-7