Abstract
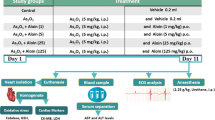
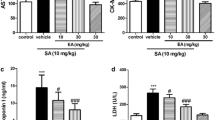
Cardiac dysfunction is one of the major causes of mortality and morbidity throughout the world. Chronic exposure of arsenic (As) mainly leads to cardiotoxic effect. Cardiotoxicity was induced by the sodium arsenite as the source of As (5 mg/kg BW, PO) for 4 weeks. As intoxication significantly (p < 0.05) increased the serum cardiac markers, viz. creatine kinase-MB, lactate dehydrogenase, aspartate transaminase, alanine transaminase and alkaline phosphatase, oxidative stress markers in heart, plasma total cholesterol (TC), triglycerides (TG), phospholipids (PL), free fatty acids (FFA), low density lipoprotein cholesterol, very low density lipoprotein cholesterol as well as cardiac lipid profile (TC, TG and FFA) and significantly (p < 0.05) decreased the level of serum high density lipoprotein cholesterol, cardiac PL, mitochondrial enzymes such as ICDH, SDH, MDH, α-KDH and NADH dehydrogenase, levels of enzymatic antioxidant, nonenzymatic antioxidants and membrane-bound ATPases in heart. In addition, As-intoxicated rats showed a significant (p < 0.05) up-regulation of myocardial NADPH (NOX) oxidase sub units such as NOX2 and NOX4 as well as Keap-1 and down-regulation of Nrf2 and HO-1 protein expressions. Pre-administration of silibinin (SB) (75 mg/kg BW) remarkably recovered all these altered parameters to near normalcy in As-induced cardiotoxic rat. Moreover, the light microscopic and transmission electron microscopic study further supports the protective efficacy of SB on the heart mitochondria. In conclusion, our data demonstrate that SB has a potential to extenuate the arsenic-induced cardiotoxicity and dyslipidemia in rat.
Graphical Abstract
.









Similar content being viewed by others
References
Devesa, V., Velez, D., & Montoro, R. (2008). Effect of thermal treatments on arsenic species contents in food. Food and Chemical Toxicology, 46, 1–8.
Vieira, C., Morais, S., Ramos, S., Delerue-Matos, C., & Oliveira, M. B. (2011). Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: Intra- and inter-specific variability and human health risks for consumption. Food and Chemical Toxicology, 49, 923–932.
Majhi, C. R., Khan, S., Leo, M. D., Manimaran, A., Sankar, P., & Sarkar, S. N. (2011). Effects of acetaminophen on reactive oxygen species and nitric oxide redox signaling in kidney of arsenic-exposed rats. Food and Chemical Toxicology, 49, 974–982.
Patra, P. H., Bandyopadhyay, S., Kumar, R., Datta, B. K., Maji, C., Biswas, S., et al. (2012). Quantitative imaging of arsenic and its species in goat following long term oral exposure. Food and chemical Toxicology, 50, 1946–1950.
Ratnaike, R. N. (2003). Acute and chronic arsenic toxicity. Postgraduate Medical Journal, 79, 391–396.
Manna, P., Sinha, M., & Sil, P. C. (2008). Arsenic induced oxidative myocardial injury: Protective role of arjunolic acid. Archives of Toxicology, 82, 137–149.
Yamauchi, H., Aminaka, Y., Yoshida, K., Sun, G., Pi, J., & Waalkes, M. P. (2004). Evaluation of DNA damage in patients with arsenic poisoning: Urinary 8-hydroxydeoxyguanine. Toxicology and Applied Pharmacology, 198, 291–296.
Tchounwou, P. B., Patlolla, A. K., & Centeno, J. A. (2003). Carcinogenic and systemic health effects associated with arsenic exposure–a critical review. Toxicologic Pathology, 31, 575–588.
Duranteau, J., Chandel, N. S., Kulisz, A., Shao, Z., & Schumacker, P. T. (1998). Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. Journal of Biological Chemistry, 273, 11619–11624.
Chang, S. I., Jin, B., Youn, P., Park, C., Park, J. D., & Ryu, D. Y. (2007). Arsenic induced toxicity and the protective role of ascorbic acid in mouse testis. Toxicology and Applied Pharmacology, 218, 196–203.
Hei, T. K., Liu, S. X., & Waldren, C. (1998). Mutagenicity of arsenic in mammalian cells: Role of reactive oxygen species. USA. Proceedings of the National Academy of Sciences, 95, 8103–8104.
Zhao, X. Y., Li, G. Y., Liu, Y., ChaiChen, J. X., Zhang, Y., Du, Z. M., et al. (2008). Resveratrol protects against arsenic trioxide induced cardiotoxicity in vitro and in vivo. British journal of pharmacology, 154, 105–113.
Shi, H., Shi, X., & Liu, K. J. (2004). Oxidative mechanism of arsenic toxicity and carcinogenesis. Molecular and Cellular Biochemistry, 255, 67–78.
Krijnen, P. A., Meischl, C., Hack, C. E., Meijer, C. J., Visser, C. A., Roos, D., et al. (2003). Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. Journal of Clinical Pathology, 56, 194–199.
Rude, M. K., Duhaney, T. A., Kuster, G. M., Judge, S., Heo, J., Colucci, W. S., et al. (2005). Aldosterone stimulates matrix metalloproteinases and reactive oxygen species in adult rat ventricular cardiomyocytes. Hypertension, 46, 555–561.
Xiao, L., Pimentel, D. R., Wang, J., Singh, K., Colucci, W. S., & Sawyer, D. B. (2002). Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. American Journal of Physiology. Cell Physiology, 282, 926–934.
Bedard, K., & Krause, K. (2007). The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiological Reviews, 87, 245–313.
Oudot, A., Vergely, C., Ecarnot-Laubriet, A., & Rochette, L. (2003). Angiotensin II activates NADPH oxidase in isolated rat hearts subjected to ischaemia-reperfusion. European Journal of Pharmacology, 462, 145–154.
Surh, Y. J. (2003). Cancer chemoprevention with dietary phytochemicals. Nature Reviews Cancer, 3, 768–780.
Kim, H., Erickson, B., Luo, W., Seward, D., Graber, J. H., Pollock, D. D., et al. (2010). Gene-specific RNA polymerase II phosphorylation and the CTD code. Nature Structural and Molecular Biology, 17, 1279–1286.
Negi, A. S., Kumar, J. K., Luqman, S., Shanker, K., Gupta, M. M., & Khanuja, S. P. S. (2008). Recent advances in plant hepatoprotectives: A chemical and biological profile of some important leads. Medicinal Research Reviews, 28, 746–772.
Muthumani, M., & Milton Prabu, S. (2012). Silibinin potentially protects arsenic-induced oxidative hepatic dysfunction in rats. Toxicology Mechanisms and Methods, 22(4), 277–288.
Faulstich, H., Jahn, W., & Wieland, T. (1980). Sylibin inhibition of amatoxin uptake in the perfused rat liver. Arzneimittel-Forschung Drug Reserch, 30, 452–454.
Middleton, E., & Kandaswami, C. (1992). Effects of flavonoids on immune and inflammatory cell function. Biochemical Pharmacology, 43, 1167–1179.
Sonnenbichler, J., & Zetl, I. (1987). Stimulating influence of a flavolignane derivative on proliferation, RNA synthesis and protein synthesis in liver cells. In I. Okolicsanyi, G. Cosmos, & G. Crepadi (Eds.), Assessment and Management of Hepatobiliary Disease (pp. 265–272). Berlin: Springer Verlag.
Mira, L., Silva, M., & Manso, C. F. (1994). Scavenging of reactive oxygen species by silibinidihemisuccinate. Biochemical Pharmacology, 48, 753–759.
Saller, R., Meier, R., & Brignoli, R. (2001). The use of silymarin in the treatment of liver diseases. Drugs, 61, 2035–2063.
Pietrangelo, A., Borella, F., Casalgrandi, G., Montosi, G., Ceccarelli, D., Gallesi, D., et al. (1995). Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology, 109, 1941–1949.
Agoston, M., Cabello, R. G., Blazovics, A., Feher, J., & Vereckei, A. (2001). The effect of aminodarone and/or antioxidant treatment on splenocyte blast transformation. Toxicology, 254, 29–41.
Kittur, S., Wilasrusmee, S., Pedersen, W. A., Mattson, M. P., Straube-West, K., Wilasrusmee, C., et al. (2002). Neurotrophic and neuroprotective effects of milk thistle (Silybum marianum) on neurons in culture. Journal of Molecular Neuroscience, 18, 265–269.
North, D. W., Gibb, H. J., & Abernathy, C. O. (1997). As: Past, present and future considerations. In C. O. Abernathy, R. L. Calderon, & W. R. Chappell (Eds.), As Exposure and Health Effects (pp. 407–423). London, UK: Chapman and Hall.
Takasawa, M., Hayakawa, M., Sugiyama, S., Hattori, K., Ito, T., & Ozawa, T. (1993). Age associated damage in mitochondrial function in rat hearts. Experimental Gerontology, 28, 269–280.
King, J. (1965). Isocitrate dehydrogenase. In: King JC, Van D, eds. Practical Clinical Enzymology. London: Nostrand Co.363.
Slater, E. C., & Borner, W. D. (1952). The effect of fluoride on the succinic oxidase system. Biochemical Journal, 52, 185–196.
Mehler, A. H., Kornberg, A., Grisolia, S., & Ochoa, S. (1948). The enzymatic mechanisms of oxidation reductions between malate or isocitrate or pyruvate. Journal of Biological Chemistry, 174, 961–977.
Reed, L. J., & Mukherjee, R. B. (1969). α-Ketoglutarate dehydrogenase complex from Escherichia coli. In J. M. Lowenstein (Ed.), Methods in Enzymology (pp. 53–61). London: Academic Press.
Minakami, S., Ringler, R. L., & Singer, T. P. (1962). Studies on the respiratory chainlinked dihydrodiphosphopyridine nucleotide dehydrogenase. I. Assay of the enzyme in particulate and in soluble preparations. Journal of Biological Chemistry, 237, 569–576.
Folch, J., Lees, M., & Sloane Stanly, G. H. (1957). A simple method for the isolation and purification of total lipids from animal tissues. Journal of Biological Chemistry, 226(1), 497–509.
Allain, C. C., Poon, L. S., Chan, C. S. G., Richmond, W., & Fu, P. C. (1974). Enzymatic determination of total serum cholesterol. Clinical Chemistry, 20(4), 470–475.
McGowan, M. W., Artiss, J. D., Strandbergh, D. R., & Zak, B. (1983). A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clinical Chemistry, 29(3), 538–542.
Zilversmit, D. B., & Davis, A. K. (1950). Micro determination of plasma phospholipids by trichloroacetic acid precipitation. Journal of Laboratory and Clinical Medicine, 35(1), 155–160.
Falholt, K., Lund, B., & Falholt, W. (1973). An easy colorimetric method for routine determination of free fatty acid in plasma. Clinica Chimica Acta, 46(2), 105–111.
Izzo, C., Grillo, F., & Murador, E. (1981). Improved method for determination of highdensity-lipoprotein cholesterol I. Isolation of high-density lipoproteins by use of polyethylene glycol 6000. Clinical Chemistry, 27(3), 371–374.
Friedwald, W. T., Levy, R. J., & Fredricken, D. S. (1972). Estimation of VLDL-cholesterol in the plasma without the use of preparative ultracentrifuge. Clinical Chemistry, 18(6), 449–502.
Niehaus, W. G, Jr, & Samuelsson, B. (1968). Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. European Journal of Biochemistry, 6, 126–130.
Jiang, Z. Y., Hunt, J. Y., & Wolff, S. P. (1992). Detection of lipid hydroperoxides using the “fox method”. Analytical Biochemistry, 202, 384–389.
Levine, R. L., Garland, D., Oliver, C. N., Amic, A., Climent, I., Lenz, A. G., et al. (1999). Determination of carbonyl content in oxidatively modified proteins. Method. Enzymol, 186, 464–478.
Rao, K. S., & Recknagel, R. O. (1968). Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Experimental and Molecular Pathology, 9, 271–278.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Arch. Biochem Biophys, 82, 70–77.
Omaye, S.T., Turbull, T.D., Sauberlich, H.C. (1979). Selected method for the determination of ascorbic acid in animal cells, tissues and fluids.In: McCormic DB, Wright DL., eds. Methods in Enzymology. New York, NY: Academic Press, 3–11.
Desai, I. D. (1984). Vitamin E analysis methods for animal tissues. Meth. Enzymol, 105, 138–147.
Kakkar, P., Das, B., & Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry & Biophysics, 21, 130–132.
Sinha, A. K. (1972). Colorimetric assay of catalase. Analytical Biochemistry, 47, 389–394.
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., & Hoekstra, W. G. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179, 588–590.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249, 7130–7139.
Horn, H. D., & Burns, F. H. (1978). Assay of glutathione reductase activity. In H. V. Bergmeyer (Ed.), Methods of Enzymatic Analysis (pp. 142–146). New York: Academic Press.
Beutler, E. (1983). Active transport of glutathione disulfide from erythrocytes. In: Larson A, Orrenius S, Holmgren A, Mannerwik BEDS., eds. Functions of Glutathione, Biochemical, Physiological, Toxicological and Clinical Aspects. New York, NY: Raven Press, 65.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., & Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 193, 265–275.
Bonting, S. L. (1970). Membrane and ion transport, presence of enzyme systems in mammalian tissues (pp. 257–263). London, UK: Wiley Interscience.
Ohnishi, T., Suzuki, T., Suzuki, Y., & Ozawa, K. (1982). A comparative study of plasma membrane Mg2 + -ATPase activities in normal, regenerating and malignant cells. Biochimica et Biophysica Acta, 684, 67–74.
Hjertén, S., & Pan, H. (1983). Purification and characterization of two forms of a low-affinity Ca2 + -ATPase from erythrocyte membranes. Biochimica et Biophysica Acta, 728, 281–288.
Basiglio, C. L., Sanchez Pozzi, E. J., Mottino, A. D., & Roma, M. G. (2009). Differential effects of silymarin and its active component SB on plasma membrane stability and hepatocellular lysis. Chemico-Biol. Interact, 179, 297–303.
Ramanathan, K., Shila, S., Kumaran, S., & Panneerselvam, C. (2003). Ascorbic acid and α-tocopherol as potent modulators on arsenic induced toxicity in mitochondria. The journal of nutritional biochemistry, 14(7), 416–420.
Chattopadhyay, S., Maiti, S., Maji, G., Deb, B., Pan, B., & Ghosh, D. (2011). Protective role of Moringa oleifera (Sajina) seed on arsenic-induced hepatocellular degeneration in female albino rats. Biological Trace Element Research, 142, 200–212.
Mccarty, M. F. (2005). Potential utility of natural polyphenols for reversing fat-induced insulin resistance. Medical Hypotheses, 64(3), 628–635.
Radhiga, T., Rajamanickam, C., Senthil, S., & Pugalendi, K. V. (2012). Effect of ursolic acid on cardiac marker enzymes, lipid profile and macrosco. Food and Chemical Toxicology, 50, 3971–3977.
Haddad, Y., Vallerand, D., Brault, A., & Haddad, P. S. (2009). Antioxidant and hepatoprotective effects of SB in a rat modal of nonalcoholic steatohepatitis. Neprology, 164, 1–11.
Yamanaka, K., Hasegawa, A., Sawamura, R., & Okada, S. (1991). Cellular response to oxidative damage in lung induced by administration of dimethyl arsenic acid, a major metabolite of inorganic arsenic in mice. Toxicology and Applied Pharmacology, 108, 205–213.
Hill, M. F., & Singal, P. K. (1997). Right and left myocardial antioxidant responses during heart failure subsequent to myocardial infarction. Circulation, 96, 2414–2420.
Forgione, M. A., Cap, A., Liao, R., et al. (2002). Heterozygous cellular glutathione peroxidase deficiency in the mouse: Abnormalities in vascular and cardiac function and structure. Circulation, 106, 1154–1158.
Li, S., Li, X., & Rozanski, G. J. (2003). Regulation of glutathione in cardiac myocytes. Journal of Molecular and Cellular Cardiology, 35, 1145–1152.
Rajnarayana, K., Sripalreddy, M., Chaluvadi, M. R., & Krishna, D. R. (2001). Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian journal of pharmacology, 33, 2–16.
Smith, K. R., & Klei, L. R. (2001). Barchowsky A. Arsenite stimulates plasma membrane NADPH oxidase in vascular endothelial cells. American Journal of Physiology. Lung Cellular and Molecular Physiology, 280, 442–449.
Acknowledgments
Authors extend their thanks to the Professor and Head, Department of Zoology and UGC-SAP for their generous support in this study.
Conflict of interest
The authors declare that there is no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muthumani, M., Prabu, S.M. Silibinin potentially attenuates arsenic-induced oxidative stress mediated cardiotoxicity and dyslipidemia in rats. Cardiovasc Toxicol 14, 83–97 (2014). https://doi.org/10.1007/s12012-013-9227-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-013-9227-x