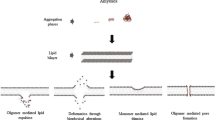
Abstract
Aberrant folded proteins and peptides are hallmarks of amyloidogenic diseases. However, the molecular processes that cause these proteins to adopt non-native structures in vivo and become cytotoxic are still largely unknown, despite intense efforts to establish a general molecular description of their behavior. Clearly, the fate of these proteins is ultimately linked to their immediate biochemical environment in vivo. In this review, we focus on the role of biological membranes, reactive interfaces that not only affect the conformational stability of amyloidogenic proteins, but also their aggregation rates and, probably, their toxicity. We first provide an overview of recent work, starting with findings regarding the amphiphatic amyloid-β protein (Aβ), which give evidence that membranes can directly promote aggregation, and that the effectiveness in this process can be related to the presence of specific neuronal ganglioside lipids. In addition, we discuss the implications of recent research (medin as an detailed example) regarding putative roles of membranes in the misfolding behavior of soluble, non-amphiphatic proteins, which are attracting increasing interest. The potential role of membranes in exerting the toxic action of misfolded proteins will also be highlighted in a molecular context. In this review, we discuss novel NMR-based approaches for exploring membrane–protein interactions, and findings obtained using them, which we use to develop a molecular concept to describe membrane-mediated protein misfolding as a quasi-two-dimensional process rather than a three-dimensional event in a biochemical environment. The aim of the review is to provide researchers with a general understanding of the involvement of membranes in folding/misfolding processes in vivo, which might be quite universal and important for future research concerning amyloidogenic and misfolding proteins, and possible ways to prevent their toxic actions.





Similar content being viewed by others
References
Masters, C. L., Simms, G., Weinman, N. A., Multhaup, G., McDonald, B. L., & Beyreuther, K. (1985). Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proceedings of the National Academy of Sciences of the United States of America, 82, 4245–4249.
Haass, C., & Selkoe, D. J. (1993). Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell, 75, 1039–1042.
Iversen, L. L., Mortishire-Smith, R. J., Pollack, S. J., & Shearman, M. S. (1995). The toxicity in vitro of beta-amyloid protein. Biochemical Journal, 311(Pt 1), 1–16.
Rochet, J. C., & Lansbury, P. T., Jr. (2000). Amyloid fibrillogenesis: Themes and variations. Current Opinion in Structural Biology, 10, 60–68.
Bucciantini, M., Giannoni, E., Chiti, F., Baroni, F., Formigli, L., Zurdo, J., et al. (2002). Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature, 416, 507–511.
Glabe, C. G. (2006). Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiology of Aging, 27, 570–575.
Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W., et al. (2003). Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science, 300, 486–489.
Petkova, A. T., Leapman, R. D., Guo, Z. H., Yau, W. M., Mattson, M. P., & Tycko, R. (2005). Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science, 307, 262–265.
Walsh, D. M., Hartley, D. M., Kusumoto, Y., Fezoui, Y., Condron, M. M., Lomakin, A., et al. (1999). Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. Journal of Biological Chemistry, 274, 25945–25952.
Klein, W. L., Stine, W. B., Jr., & Teplow, D. B. (2004). Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiology of Aging, 25, 569–580.
Haass, C., & Selkoe, D. J. (2007). Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nature Reviews. Molecular Cell Biology, 8, 101–112.
Chimon, S., & Ishii, Y. (2005). Capturing intermediate structures of Alzheimer’s beta-amyloid, Abeta(1–40), by solid-state NMR spectroscopy. Journal of the American Chemical Society, 127, 13472–13473.
Zetterstrom, P., Stewart, H. G., Bergemalm, D., Jonsson, P. A., Graffmo, K. S., Andersen, P. M., et al. (2007). Soluble misfolded subfractions of mutant superoxide dismutase-1s are enriched in spinal cords throughout life in murine ALS models. Proceedings of the National Academy of Sciences of the United States of America, 104, 14157–14162.
Velde, C. V., Miller, T. M., Cashman, N. R., & Cleveland, D. W. (2008). Selective association of misfolded ALS-linked mutant SOD1 with the cytoplasmic face of mitochondria. Proceedings of the National Academy of Sciences of the United States of America, 105, 4022–4027.
Walsh, D. M., Tseng, B. P., Rydel, R. E., Podlisny, M. B., & Selkoe, D. J. (2000). The oligomerization of amyloid beta-protein begins intracellularly in cells derived from human brain. Biochemistry, 39, 10831–10839.
Demeester, N., Baier, G., Enzinger, C., Goethals, M., Vandekerckhove, J., Rosseneu, M., et al. (2000). Apoptosis induced in neuronal cells by C-terminal amyloid beta-fragments is correlated with their aggregation properties in phospholipid membranes. Molecular Membrane Biology, 17, 219–228.
Kawahara, M., Kuroda, Y., Arispe, N., & Rojas, E. (2000). Alzheimer’s beta-amyloid, human islet amylin, and prion protein fragment evoke intracellular free calcium elevations by a common mechanism in a hypothalamic GnRH neuronal cell line. Journal of Biological Chemistry, 275, 14077–14083.
Lin, H., Bhatia, R., & Lal, R. (2001). Amyloid beta protein forms ion channels: Implications for Alzheimer’s disease pathophysiology. FASEB Journal, 15, 2433–2444.
McLaurin, J., Yang, D., Yip, C. M., & Fraser, P. E. (2000). Review: Modulating factors in amyloid-beta fibril formation. Journal of Structural Biology, 130, 259–270.
Curtain, C. C., Ali, F. E., Smith, D. G., Bush, A. I., Masters, C. L., & Barnham, K. J. (2003). Metal ions, pH, and cholesterol regulate the interactions of Alzheimer’s disease amyloid-beta peptide with membrane lipid. Journal of Biological Chemistry, 278, 2977–2982.
Terzi, E., Holzemann, G., & Seelig, J. (1997). Interaction of Alzheimer beta-amyloid peptide(1–40) with lipid membranes. Biochemistry, 36, 14845–14852.
Simons, M., Keller, P., De Strooper, B., Beyreuther, K., Dotti, C. G., & Simons, K. (1998). Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proceedings of the National Academy of Sciences of the United States of America, 95, 6460–6464.
Scheuermann, S., Hambsch, B., Hesse, L., Stumm, J., Schmidt, C., Beher, D., et al. (2001). Homodimerization of amyloid precursor protein and its implication in the amyloidogenic pathway of Alzheimer’s disease. Journal of Biological Chemistry, 276, 33923–33929.
Engel, M. F. M., Khemtemourian, L., Kleijer, C. C., Meeldijk, H. J. D., Jacobs, J., Verkleij, A. J., et al. (2008). Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proceedings of the National Academy of Sciences of the United States of America, 105, 6033–6038.
Shai, Y. (2002). Mode of action of membrane active antimicrobial peptides. Biopolymers, 66, 236–248.
Shai, Y. (1999). Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochimica et Biophysica Acta, 1462, 55–70.
Bonev, B. B., Chan, W. C., Bycroft, B. W., Roberts, G. C., & Watts, A. (2000). Interaction of the lantibiotic nisin with mixed lipid bilayers: A 31P and 2H NMR study. Biochemistry, 39, 11425–11433.
Wieprecht, T., Apostolov, O., Beyermann, M., & Seelig, J. (2000). Membrane binding and pore formation of the antibacterial peptide PGLa: Thermodynamic and mechanistic aspects. Biochemistry, 39, 442–452.
Seelig, J. (2004). Thermodynamics of lipid-peptide interactions. Biochimica et Biophysica Acta – Biomembranes, 1666, 40–50.
Giacomelli, C. E., & Norde, W. (2005). Conformational changes of the amyloid beta-peptide (1–40) adsorbed on solid surfaces. Macromolecular Bioscience, 5, 401–407.
Bokvist, M., Lindstrom, F., Watts, A., & Grobner, G. (2004). Two types of Alzheimer’s beta-amyloid (1–40) peptide membrane interactions: Aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. Journal of Molecular Biology, 335, 1039–1049.
Bokvist, M., & Grobner, G. (2007). Misfolding of amyloidogenic proteins at membrane surfaces: The impact of macromolecular crowding. Journal of the American Chemical Society, 129, 14848–14849.
Maltseva, E., Kerth, A., Blume, A., Mohwald, H., & Brezesinski, G. (2005). Adsorption of amyloid beta (1–40) peptide at phospholipid monolayers. Chembiochem, 6, 1817–1824.
Zhao, H., Tuominen, E. K., & Kinnunen, P. K. (2004). Formation of amyloid fibers triggered by phosphatidylserine-containing membranes. Biochemistry, 43, 10302–10307.
Lindstrom, F., Bokvist, M., Sparrman, T., & Grobner, G. (2002). Association of amyloid-beta peptide with membrane surfaces monitored by solid state NMR. Physical Chemistry Chemical Physics, 4, 5524–5530.
Kakio, A., Nishimoto, S., Yanagisawa, K., Kozutsumi, Y., & Matsuzaki, K. (2002). Interactions of amyloid beta-protein with various gangliosides in raft-like membranes: Importance of GM1 ganglioside-bound form as an endogenous seed for Alzheimer amyloid. Biochemistry, 41, 7385–7390.
Waschuk, S. A., Elton, E. A., Darabie, A. A., Fraser, P. E., & McLaurin, J. A. (2001). Cellular membrane composition defines A beta-lipid interactions. Journal of Biological Chemistry, 276, 33561–33568.
Gibson Wood, W., Eckert, G. P., Igbavboa, U., & Muller, W. E. (2003). Amyloid beta-protein interactions with membranes and cholesterol: Causes or casualties of Alzheimer’s disease. Biochimica et Biophysica Acta, 1610, 281–290.
Devanathan, S., Salamon, Z., Lindblom, G., Grobner, G., & Tollin, G. (2006). Effects of sphingomyelin, cholesterol and zinc ions on the binding, insertion and aggregation of the amyloid Abeta(1–40) peptide in solid-supported lipid bilayers. FEBS Journal, 273, 1389–1402.
Aisenbrey, C., Borowik, T., Bystrom, R., Bokvist, M., Lindstrom, F., Misiak, H., et al. (2008). How is protein aggregation in amyloidogenic diseases modulated by biological membranes? European Biophysics Journal, 37, 247–255.
Mandal, P. K., & Pettegrew, J. W. (2004). Alzheimer’s disease: Soluble oligomeric A beta(1–40) peptide in membrane mimic environment from solution NMR and circular dichroism studies. Neurochemical Research, 29, 2267–2272.
Terzi, E., Holzemann, G., & Seelig, J. (1995). Self-association of beta-amyloid peptide(1–40) in solution and binding to lipid-membranes. Journal of Molecular Biology, 252, 633–642.
Simakova, O., & Arispe, N. J. (2006). Early and late cytotoxic effects of external application of the Alzheimer’s Abeta result from the initial formation and function of Abeta ion channels. Biochemistry, 45, 5907–5915.
Lal, R., Lin, H., & Quist, A. P. (2007). Amyloid beta ion channel: 3D structure and relevance to amyloid channel paradigm. Biochimica et Biophysica Acta, 1768, 1966–1975.
Olofsson, A., Borowik, T., Grobner, G., & Sauer-Eriksson, A. E. (2007). Negatively charged phospholipid membranes induce amyloid formation of medin via an alpha-helical intermediate. Journal of Molecular Biology, 374, 186–194.
Beyer, K. (2007). Mechanistic aspects of Parkinson’s disease: α-Synuclein and the membrane. Cell Biochemistry and Biophysics, 47, 285–299.
Lindstrom, F., Williamson, P. T. F., & Grobner, G. (2005). Molecular insight into the electrostatic membrane surface potential by N-14/P-31 MAS NMR spectroscopy: Nociceptin-lipid association. Journal of the American Chemical Society, 127, 6610–6616.
Minton, A. P. (1999). Adsorption of globular proteins on locally planar surfaces. II. Models for the effect of multiple adsorbate conformations on adsorption equilibria and kinetics. Biophysical Journal, 76, 176–187.
Seelig, J. (1997). Titration calorimetry of lipid-peptide interactions. Biochimica et Biophysica Acta—Reviews on Biomembranes, 1331, 103–116.
Murphy, R. M. (2007). Kinetics of amyloid formation and membrane interaction with amyloidogenic proteins. Biochimica et Biophysica Acta, 1768, 1923–1934.
Lau, T. L., Ambroggio, E. E., Tew, D. J., Cappai, R., Masters, C. L., Fidelio, G. D., et al. (2006). Amyloid-beta peptide disruption of lipid membranes and the effect of metal ions. Journal of Molecular Biology, 356, 759–770.
Sparr, E., Engel, M. F. M., Sakharov, D. V., Sprong, M., Jacobs, J., de Kruijff, B., et al. (2004). Islet amyloid polypeptide-induced membrane leakage involves uptake of lipids by forming amyloid fibers. FEBS Letters, 577, 117–120.
Ravault, S., Soubias, O., Saurel, O., Thomas, A., Brasseur, R., & Milon, A. (2005). Fusogenic Alzheimer’s peptide fragment Abeta (29–42) in interaction with lipid bilayers: Secondary structure, dynamics, and specific interaction with phosphatidyl ethanolamine polar heads as revealed by solid-state NMR. Protein Science, 14, 1181–1189.
Munishkina, L. A., & Fink, A. L. (2007). Fluorescence as a method to reveal structures and membrane-interactions of amyloidogenic proteins. Biochimica et Biophysica Acta, 1768, 1862–1885.
Matsuzaki, K. (2007). Physicochemical interactions of amyloid beta-peptide with lipid bilayers. Biochimica et Biophysica Acta, 1768, 1935–1942.
Yip, C. M., & McLaurin, J. (2001). Amyloid-beta peptide assembly: A critical step in fibrillogenesis and membrane disruption. Biophysical Journal, 80, 1359–1371.
Lopes, D. H., Meister, A., Gohlke, A., Hauser, A., Blume, A., & Winter, R. (2007). Mechanism of islet amyloid polypeptide fibrillation at lipid interfaces studied by infrared reflection absorption spectroscopy. Biophysical Journal, 93, 3132–3141.
Kremer, J. J., Sklansky, D. J., & Murphy, R. M. (2001). Profile of changes in lipid bilayer structure caused by beta-amyloid peptide. Biochemistry, 40, 8563–8571.
Barnham, K. J., Ciccotosto, G. D., Tickler, A. K., Ali, F. E., Smith, D. G., Williamson, N. A., et al. (2003). Neurotoxic, redox-competent Alzheimer’s beta-amyloid is released from lipid membrane by methionine oxidation. Journal of Biological Chemistry, 278, 42959–42965.
Esposito, C., Tedeschi, A., Scrima, M., D’Errico, G., Ottaviani, M. F., Rovero, P., et al. (2006). Exploring interaction of beta-amyloid segment (25–35) with membrane models through paramagnetic probes. Journal of Peptide Science, 12, 766–774.
Lau, T. L., Gehman, J. D., Wade, J. D., Perez, K., Masters, C. L., Barnham, K. J., et al. (2007). Membrane interactions and the effect of metal ions of the amyloidogenic fragment Abeta(25–35) in comparison to Abeta(1–42). Biochimica et Biophysica Acta, 1768, 2400–2408.
Veatch, S. L., Leung, S. S., Hancock, R. E., & Thewalt, J. L. (2007). Fluorescent probes alter miscibility phase boundaries in ternary vesicles. Journal of Physical Chemistry B, 111, 502–504.
Seelig, J. (1978). 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochimica et Biophysica Acta, 515, 105–140.
Cullis, P. R., & de Kruijff, B. (1979). Lipid polymorphism and the functional roles of lipids in biological membranes. Biochimica et Biophysica Acta, 559, 399–420.
Bechinger, B., & Seelig, J. (1991). Interaction of electric dipoles with phospholipid head groups—A H-2 and P-31 NMR-study of phloretin and phloretin analogs in phosphatidylcholine membranes. Biochemistry, 30, 3923–3929.
Macdonald, P. M., Leisen, J., & Marassi, F. M. (1991). Response of phosphatidylcholine in the gel and liquid-crystalline states to membrane surface charges. Biochemistry, 30, 3558–3566.
Roux, M., Neumann, J. M., Hodges, R. S., Devaux, P. F., & Bloom, M. (1989). Conformational changes of phospholipid headgroups induced by a cationic integral membrane peptide as seen by deuterium magnetic resonance. Biochemistry, 28, 2313–2321.
Sixl, F., & Watts, A. (1983). Headgroup interactions in mixed phospholipid bilayers. Proceedings of the National Academy of Sciences of the United States of America, 80, 1613–1615.
Pinheiro, T. J., & Watts, A. (1994). Resolution of individual lipids in mixed phospholipid membranes and specific lipid-cytochrome c interactions by magic-angle spinning solid-state phosphorus-31 NMR. Biochemistry, 33, 2459–2467.
Carbone, M. A., & Macdonald, P. M. (1996). Cardiotoxin II segregates phosphatidylglycerol from mixtures with phosphatidylcholine: (31)P and (2)H NMR spectroscopic evidence. Biochemistry, 35, 3368–3378.
Bonev, B. B., Breukink, E., Swiezewska, E., de Kruijff, B., & Watts, A. (2004). Targeting extracellular pyrophosphates underpins the high selectivity of nisin. FASEB Journal, 18, 1862–1869.
Seelig, J., Lehrmann, R., & Terzi, E. (1995). Domain formation induced by lipid ion and lipid peptide interactions. Molecular Membrane Biology, 12, 51–57.
Smith, D. P., Smith, D. G., Curtain, C. C., Boas, J. F., Pilbrow, J. R., Ciccotosto, G. D., et al. (2006). Copper-mediated amyloid-beta toxicity is associated with an intermolecular histidine bridge. Journal of Biological Chemistry, 281, 15145–15154.
Lau, T. L., Gehman, J. D., Wade, J. D., Masters, C. L., Barnham, K. J., & Separovic, F. (2007). Cholesterol and clioquinol modulation of A beta(1–42) interaction with phospholipid bilayers and metals. Biochimica et Biophysica Acta, 1768, 3135–3144.
Caragounis, A., Du, T., Filiz, G., Laughton, K. M., Volitakis, I., Sharples, R. A., et al. (2007). Differential modulation of Alzheimer’s disease amyloid beta-peptide accumulation by diverse classes of metal ligands. Biochemical Journal, 407, 435–450.
Mandal, P. K., Pettegrew, J. W., Mckeag, D. W., & Mandal, R. (2006). Alzheimer’s disease: Halothane induces A beta peptide to oligomeric form-solution NMR studies. Neurochemical Research, 31, 883–890.
Chauhan, A., Ray, I., & Chauhan, V. P. (2000). Interaction of amyloid beta-protein with anionic phospholipids: Possible involvement of Lys28 and C-terminus aliphatic amino acids. Neurochemical Research, 25, 423–429.
Mobley, D. L., Cox, D. L., Singh, R. R., Maddox, M. W., & Longo, M. L. (2004). Modeling amyloid beta-peptide insertion into lipid bilayers. Biophysical Journal, 86, 3585–3597.
Rangachari, V., Reed, D. K., Moore, B. D., & Rosenberry, T. L. (2006). Secondary structure and interfacial aggregation of amyloid-beta(1–40) on sodium dodecyl sulfate micelles. Biochemistry, 45, 8639–8648.
Coles, M., Bicknell, W., Watson, A. A., Fairlie, D. P., & Craik, D. J. (1998). Solution structure of amyloid beta-peptide(1–40) in a water-micelle environment. Is the membrane-spanning domain where we think it is? Biochemistry, 37, 11064–11077.
Ji, S. R., Wu, Y., & Sui, S. F. (2002). Cholesterol is an important factor affecting the membrane insertion of beta-amyloid peptide (A beta 1–40), which may potentially inhibit the fibril formation. Journal of Biological Chemistry, 277, 6273–6279.
Kracun, I., Kalanj, S., Cosovic, C., & Talan-Hranilovic, J. (1990). Brain gangliosides in Alzheimer’s disease. Journal für Hirnforschung, 31, 789–793.
Kracun, I., Kalanj, S., Talan-Hranilovic, J., & Cosovic, C. (1992). Cortical distribution of gangliosides in Alzheimer’s disease. Neurochemistry International, 20, 433–438.
Cordy, J. M., Hooper, N. M., & Turner, A. J. (2006). The involvement of lipid rafts in Alzheimer’s disease. Molecular Membrane Biology, 23, 111–122.
Williamson, R., Usardi, A., Hanger, D. P., & Anderton, B. H. (2008). Membrane-bound beta-amyloid oligomers are recruited into lipid rafts by a fyn-dependent mechanism. FASEB Journal, 22, 1552–1559.
Yanagisawa, K. (2007). Role of gangliosides in Alzheimer’s disease. Biochimica et Biophysica Acta, 1768, 1943–1951.
McLaurin, J., & Chakrabartty, A. (1996). Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. Journal of Biological Chemistry, 271, 26482–26489.
McLaurin, J., Franklin, T., Fraser, P. E., & Chakrabartty, A. (1998). Structural transitions associated with the interaction of Alzheimer beta-amyloid peptides with gangliosides. Journal of Biological Chemistry, 273, 4506–4515.
Williamson, M. P., Suzuki, Y., Bourne, N. T., & Asakura, T. (2006). Binding of amyloid beta-peptide to ganglioside micelles is dependent on histidine-13. Biochemical Journal, 397, 483–490.
Choo-Smith, L. P., Garzon-Rodriguez, W., Glabe, C. G., & Surewicz, W. K. (1997). Acceleration of amyloid fibril formation by specific binding of Abeta-(1–40) peptide to ganglioside-containing membrane vesicles. Journal of Biological Chemistry, 272, 22987–22990.
Choo-Smith, L. P., & Surewicz, W. K. (1997). The interaction between Alzheimer amyloid beta(1–40) peptide and ganglioside GM1-containing membranes. FEBS Letters, 402, 95–98.
Ariga, T., Kobayashi, K., Hasegawa, A., Kiso, M., Ishida, H., & Miyatake, T. (2001). Characterization of high-affinity binding between gangliosides and amyloid beta-protein. Archives of Biochemistry and Biophysics, 388, 225–230.
Chi, E. Y., Frey, S. L., & Lee, K. Y. (2007). Ganglioside G(M1)-mediated amyloid-beta fibrillogenesis and membrane disruption. Biochemistry, 46, 1913–1924.
Matsuzaki, K., & Horikiri, C. (1999). Interactions of amyloid beta-peptide (1–40) with ganglioside-containing membranes. Biochemistry, 38, 4137–4142.
Kakio, A., Nishimoto, S. I., Yanagisawa, K., Kozutsumi, Y., & Matsuzaki, K. (2001). Cholesterol-dependent formation of GM1 ganglioside-bound amyloid beta-protein, an endogenous seed for Alzheimer amyloid. Journal of Biological Chemistry, 276, 24985–24990.
Okada, T., Wakabayashi, M., Ikeda, K., & Matsuzaki, K. (2007). Formation of toxic fibrils of Alzheimer’s amyloid beta-protein-(1–40) by monosialoganglioside GM1, a neuronal membrane component. Journal of Molecular Biology, 371, 481–489.
Wakabayashi, M., & Matsuzaki, K. (2007). Formation of amyloids by Abeta-(1–42) on NGF-differentiated PC12 cells: Roles of gangliosides and cholesterol. Journal of Molecular Biology, 371, 924–933.
Mandal, P. K., & Pettegrew, J. W. (2004). Alzheimer’s disease: NMR studies of asialo (GM1) and trisialo (GT1b) ganglioside interactions with Abeta(1–40) peptide in a membrane mimic environment. Neurochemical Research, 29, 447–453.
Fernandez, A., & Berry, R. S. (2003). Proteins with H-bond packing defects are highly interactive with lipid bilayers: Implications for amyloidogenesis. Proceedings of the National Academy of Sciences of the United States of America, 100, 2391–2396.
Fernandez, A., Kardos, J., Scott, L. R., Goto, Y., & Berry, R. S. (2003). Structural defects and the diagnosis of amyloidogenic propensity. Proceedings of the National Academy of Sciences of the United States of America, 100, 6446–6451.
Alakoskela, J. M., Jutila, A., Simonsen, A. C., Pirneskoski, J., Pyhajoki, S., Turunen, R., et al. (2006). Characteristics of fibers formed by cytochrome c and induced by anionic phospholipids. Biochemistry, 45, 13447–13453.
Gorbenko, G. P., & Kinnunen, P. K. (2006). The role of lipid-protein interactions in amyloid-type protein fibril formation. Chemistry and Physics of Lipids, 141, 72–82.
Mandal, P. K., Pettegrew, J. W., Masliah, E., Hamilton, R. L., & Mandal, R. (2006). Interaction between A beta peptide and alpha synuclein: Molecular mechanisms in overlapping pathology of Alzheimer’s and Parkinson’s in dementia with Lewy body disease. Neurochemical Research, 31, 1153–1162.
Brender, J. R., Durr, U. H., Heyl, D., Budarapu, M. B., & Ramamoorthy, A. (2007). Membrane fragmentation by an amyloidogenic fragment of human Islet Amyloid Polypeptide detected by solid-state NMR spectroscopy of membrane nanotubes. Biochimica et Biophysica Acta, 1768, 2026–2029.
Khemtemourian, L., Killian, J. A., Hoppener, J. W., & Engel, M. F. (2008). Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in beta-cell death in type 2 diabetes mellitus. Experimental Diabetes Research, 2008, 421287.
Bruijn, L. I., Houseweart, M. K., Kato, S., Anderson, K. L., Anderson, S. D., Ohama, E., et al. (1998). Aggregation and motor neuron toxicity of an ALS-linked SOD1 mutant independent from wild-type SOD1. Science, 281, 1851–1854.
Lindberg, M. J., Bystrom, R., Boknas, N., Andersen, P. M., & Oliveberg, M. (2005). Systematically perturbed folding patterns of amyotrophic lateral sclerosis (ALS)-associated SOD1 mutants. Proceedings of the National Academy of Sciences of the United States of America, 102, 9754–9759.
Liu, J., Lillo, C., Jonsson, P. A., Velde, C. V., Ward, C. M., Miller, T. M., et al. (2004). Toxicity of familial ALS-linked SOD1 mutants from selective recruitment to spinal mitochondria. Neuron, 43, 5–17.
Haggqvist, B., Naslund, J., Sletten, K., Westermark, G. T., Mucchiano, G., Tjernberg, L. O., et al. (1999). Medin: An integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proceedings of the National Academy of Sciences of the United States of America, 96, 8669–8674.
Ceriani, R. L., Thompson, K., Peterson, J. A., & Abraham, S. (1977). Surface differentiation antigens of human mammary epithelial cells carried on the human milk fat globule. Proceedings of the National Academy of Sciences of the United States of America, 74, 582–586.
Hanayama, R., Tanaka, M., Miwa, K., Shinohara, A., Iwamatsu, A., & Nagata, S. (2002). Identification of a factor that links apoptotic cells to phagocytes. Nature, 417, 182–187.
Shi, J., Heegaard, C. W., Rasmussen, J. T., & Gilbert, G. E. (2004). Lactadherin binds selectively to membranes containing phosphatidyl-L-serine and increased curvature. Biochimica et Biophysica Acta, 1667, 82–90.
Shi, J., & Gilbert, G. E. (2003). Lactadherin inhibits enzyme complexes of blood coagulation by competing for phospholipid-binding sites. Blood, 101, 2628–2636.
Taylor, M. R., Couto, J. R., Scallan, C. D., Ceriani, R. L., & Peterson, J. A. (1997). Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA and Cell Biology, 16, 861–869.
Larsson, A., Peng, S., Wassberg, E., Gerwins, P., Thelin, S., & Westermark, P. (2006). Medin and medin-amyloid in thoracic aortic aneurysm and dissection. Amyloid—Journal of Protein Folding Disorders, 13, 40.
Larsson, A., Peng, S., Persson, H., Rosenbloom, J., Abrams, W. R., Wassberg, E., et al. (2006). Lactadherin binds to elastin—A starting point for medin amyloid formation? Amyloid—Journal of Protein Folding Disorders, 13, 78–85.
Larsson, A., Soderberg, L., Westermark, G. T., Sletten, K., Engstrom, U., Tjernberg, L. O., et al. (2007). Unwinding fibril formation of medin, the peptide of the most common form of human amyloid. Biochemical and Biophysical Research Communications, 361, 822–828.
Knight, J. D., Hebda, J. A., & Miranker, A. D. (2006). Conserved and cooperative assembly of membrane-bound alpha-helical states of islet amyloid polypeptide. Biochemistry, 45, 9496–9508.
Fezoui, Y., & Teplow, D. B. (2002). Kinetic studies of amyloid beta-protein fibril assembly. Differential effects of alpha-helix stabilization. Journal of Biological Chemistry, 277, 36948–36954.
Munishkina, L. A., Phelan, C., Uversky, V. N., & Fink, A. L. (2003). Conformational behavior and aggregation of alpha-synuclein in organic solvents: Modeling the effects of membranes. Biochemistry, 42, 2720–2730.
Kirkitadze, M. D., Condron, M. M., & Teplow, D. B. (2001). Identification and characterization of key kinetic intermediates in amyloid beta-protein fibrillogenesis. Journal of Molecular Biology, 312, 1103–1119.
Barnham, K. J., Cappai, R., Beyreuther, K., Masters, C. L., & Hill, A. F. (2006). Delineating common molecular mechanisms in Alzheimer’s and prion diseases. Trends in Biochemical Sciences, 31, 465–472.
Ehrnhoefer, D. E., Bieschke, J., Boeddrich, A., Herbst, M., Masino, L., Lurz, R., et al. (2008). EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nature Structural & Molecular Biology, 15, 558–566.
Chromy, B. A., Nowak, R. J., Lambert, M. P., Viola, K. L., Chang, L., Velasco, P. T., et al. (2003). Self-assembly of Abeta(1–42) into globular neurotoxins. Biochemistry, 42, 12749–12760.
Catalano, S. M., Dodson, E. C., Henze, D. A., Joyce, J. G., Krafft, G. A., & Kinney, G. G. (2006). The role of amyloid-beta derived diffusible ligands (ADDLs) in Alzheimer’s disease. Current Topics in Medicinal Chemistry, 6, 597–608.
Hepler, R. W., Grimm, K. M., Nahas, D. D., Breese, R., Dodson, E. C., Acton, P., et al. (2006). Solution state characterization of amyloid beta-derived diffusible ligands. Biochemistry, 45, 15157–15167.
Surolia, I., Sarkar, D. P., & Sinha, S. (2008). Form and dimensions of aggregates dictate cytotoxicities of Danish dementia peptides. Biochemical and Biophysical Research Communications, 372, 62–66.
de Planque, M. R., Raussens, V., Contera, S. A., Rijkers, D. T., Liskamp, R. M., Ruysschaert, J. M., et al. (2007). Beta-sheet structured beta-amyloid(1–40) perturbs phosphatidylcholine model membranes. Journal of Molecular Biology, 368, 982–997.
Snyder, E. M., Nong, Y., Almeida, C. G., Paul, S., Moran, T., Choi, E. Y., et al. (2005). Regulation of NMDA receptor trafficking by amyloid-beta. Nature Neuroscience, 8, 1051–1058.
Langmuir, I. (1916). The constitution and fundamental properties of solids and liquids. Part I. Solids. Journal of the American Chemical Society, 38, 2221–2295.
Beta, C., Moula, M. G., Mikhailov, A. S., Rotermund, H. H., & Ertl, G. (2004). Excitable CO oxidation on Pt(110) under nonuniform coupling. Physical Review Letters, 93, 188302.
White, S. H., & Wimley, W. C. (1999). Membrane protein folding and stability: Physical principles. Annual Review of Biophysics and Biomolecular Structure, 28, 319–365.
Minton, A. P. (2006). Macromolecular crowding. Current Biology, 16, R269–R271.
Talbot, J., Jin, X., & Wang, N. H. L. (1994). New equations for multicomponent adsorption-kinetics. Langmuir, 10, 1663–1666.
Chatelier, R. C., & Minton, A. P. (1996). Adsorption of globular proteins on locally planar surfaces: Models for the effect of excluded surface area and aggregation of adsorbed protein on adsorption equilibria. Biophysical Journal, 71, 2367–2374.
Aisenbrey, C., Bechinger, B., & Grobner, G. (2008). Macromolecular crowding at membrane interfaces: Adsorption and alignment of membrane peptides. Journal of Molecular Biology, 375, 376–385.
Sieber, J. J., Willig, K. I., Kutzner, C., Gerding-Reimers, C., Harke, B., Donnert, G., et al. (2007). Anatomy and dynamics of a supramolecular membrane protein cluster. Science, 317, 1072–1076.
Ellis, R. J. (2001). Macromolecular crowding: Obvious but underappreciated. Trends in Biochemical Sciences, 26, 597–604.
Heimburg, T., & Jackson, A. D. (2005). On soliton propagation in biomembranes and nerves. Proceedings of the National Academy of Sciences of the United States of America, 102, 9790–9795.
Raskin, D. M., & de Boer, P. A. J. (1999). MinDE-dependent pole-to-pole oscillation of division inhibitor MinC in Escherichia coli. Journal of Bacteriology, 181, 6419–6424.
Durell, S. R., Guy, H. R., Arispe, N., Rojas, E., & Pollard, H. B. (1994). Theoretical models of the ion channel structure of amyloid beta-protein. Biophysical Journal, 67, 2137–2145.
Acknowledgments
This work was supported by the Knut and Alice Wallenberg Foundation, Swedish Research Council (NT and Medicine), Umeå University Biotechnology Fund, Insamlingsstiftelse, Magn. Bergvalls Foundation, Carl Trygger Foundation, Alzheimerfonden, Socialstyrelsen, Hjärnfonden, Åke Wibergs Foundation, Göran Gustafssons Foundation, the Swedish Research Science Council, Ernst Schering Foundation, Centre for Biomedical Engineering at Wrocław University of Technology and the patients’ association FAMY/AMYL. We thank M. Oliveberg, S. Marklund, E. Sauer-Eriksson, G. Lindblom, L. Johansson, and E. Rosenbaum for all their support.
Author information
Authors and Affiliations
Corresponding author
Appendix: Basics of Protein Membrane Adsorption
Appendix: Basics of Protein Membrane Adsorption
Surface physics and surface chemistry are well-established fields that have made major contributions to theoretical descriptions and practical applications of physico-chemical phenomena in physics and chemistry. Surfaces can provide templates for both physical and chemical processes (e.g., adsorption and catalytic reactions, respectively; [130, 131]) and can have highly specific properties due to their 2D nature. Traditionally, the main role of cells’ lipid membranes is separating intracellular compartments and the cell interior from its environment (thus maintaining cellular integrity). In this context, the membrane proteins play key roles in controlling the exchange of molecules and information. However, lipid membranes also provide large surface areas (relative to their enclosed volumes), and the lipid membrane surface area is close to maximal in some organelles (e.g., the endoplasmic reticulum, Golgi apparatus, and mitochondria). Hence, the specific properties of the membrane surfaces have the potential to play additional roles in cellular processes. It is, therefore, worthwhile to explore the role of lipid membranes as two-dimensional surface templates that facilitate cellular processes.
Adsorption Isotherms: Protein Surface Crowding
One possible approach to describe the adsorption of molecules to the membrane surface is to apply the equation for the intermolecular binding equilibrium in the form of [132]:
where [PL], [P], and [L] are the concentrations of lipid bound protein, free protein, and lipid, respectively. However, the description of intermolecular binding is poorly compatible with the process of molecule adsorption onto membrane. The equation fits only into a molecular picture, when the lipid concentration [L] is considered as the membrane surface area per volume. Also, in most cases intermolecular interactions between the adsorbed molecules are not negligible during surface adsorption.
Langmuir described the special properties of surfaces in his fundamental essay about “The constitution and fundamental properties of solids and liquids” [130] at the beginning of the last century. His description of adsorption of gases by solids, based on kinetic arguments (adsorption and desorption rates) are still the basis of surface physics.
where Θ: surface coverage; α: constant; and P: gas pressure.
The equation reflects the necessity to consider intermolecular interactions on surfaces by introducing a surface coverage. The theory, however, idealizes intermolecular interactions by assuming an average occupancy of the surface per adsorbed molecule. But in a highly populated environment, the effective free space of a molecule is much lower than the average free space, due to the crowding effect [133].
For many processes, a more detailed description of the intermolecular interactions of adsorbed and free molecules is essential for any deeper mechanistic understanding. An analytic description of the membrane occupation, for example by a scaled particle theory [134], leads to a description of the effect of crowding on different adsorbed states [135, 136]. Notably the high surface occupancy favors the formation of multimeric states (Figs. 1 and 5). The analytical results were confirmed by experiments and computer simulations [137] for syntaxin 1, a protein involved in the initiation of membrane fusion [138].
However, the cell interior by itself with its high protein content of up to 200–300 g/l provides a crowded environment, which additionally affects the adsorption into the crowded membrane surface [32]. Here, the effect of crowding in the bulk volume on the adsorption of the amyloid Aβ has been demonstrated by the addition of ficoll, an inert macromolecule. In general, membrane surfaces can act as templates in numerous biological processes, ranging from electro-mechanical waves coupled to nerve pulse transmission to oscillating partition systems involved in cell division of Escherichia coli bacteria [131, 139, 140].
Rights and permissions
About this article
Cite this article
Byström, R., Aisenbrey, C., Borowik, T. et al. Disordered Proteins: Biological Membranes as Two-Dimensional Aggregation Matrices. Cell Biochem Biophys 52, 175–189 (2008). https://doi.org/10.1007/s12013-008-9033-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-008-9033-4