Abstract
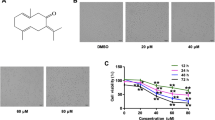
In this study, the anti-proliferative effect of physcion, an anthraquinone derivative isolated and characterized from both terrestrial and marine sources, against human gastric cancer SGC-7901 cells was investigated and the underlying mechanisms were explored. Physcion reduced SGC-7901 cell viability in a dose- and time-dependent manner, as demonstrated by MTT assay. It triggered the mitochondrial/caspase apoptotic pathway indicated by loss of mitochondrial membrane potential and cytochrome c release. Moreover, physcion induced a sustained activation of the phosphorylation of AMPK, and compound C (an inhibitor of AMPK) significantly reversed physcion-induced apoptosis in SGC-7901 cells. In addition, physcion provoked the generation of reactive oxygen species (ROS) in SGC-7901 cells, while the antioxidant N-acetyl cysteine almost completely blocked physcion-induced AMPK activation and apoptosis. Taken together, these findings suggest that physcion induces apoptosis through a ROS/AMPK-dependent mitochondrial pathway.







Similar content being viewed by others
References
Jemal, A., et al. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 61(2), 69–90.
Agarwal, S. K., et al. (2000). Antifungal activity of anthraquinone derivatives from Rheum emodi. Journal of Ethnopharmacology, 72(1–2), 43–46.
Anke, H., et al. (1980). Metabolic products of microorganisms. 185. The anthraquinones of the Aspergillus glaucus group. I. Occurrence, isolation, identification and antimicrobial activity. Archives of Microbiology, 126(3), 223–230.
Smetanina, O. F., et al. (2007). Indole alkaloids produced by a marine fungus isolate of Penicillium janthinellum Biourge. Journal of Natural Products, 70(6), 906–909.
Zhao, Y. L., et al. (2009). Investigations of free anthraquinones from rhubarb against alpha-naphthylisothiocyanate-induced cholestatic liver injury in rats. Basic & Clinical Pharmacology & Toxicology, 104(6), 463–469.
Ghosh, S., et al. (2010). Anti-inflammatory and anticancer compounds isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn. Journal of Pharmacy and Pharmacology, 62(9), 1158–1166.
Tamokou Jde, D., Tala, M. F., Wabo, H. K., Kuiate, J. R., & Tane, P. (2009). Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. Journal of Ethnopharmacology, 124(3), 571–575.
Almeida, A. P., Dethoup, T., Singburaudom, N., Lima, R., Vasconcelos, M. H., & Pinto, M. (2010). The in vitro anticancer activity of the crude extract of the sponge-associated fungus Eurotium cristatum and its secondary metabolites. Journal of natural Pharmaceuticals, 1(1), 25–29.
Chen, G., et al. (2012). Synergistic antitumor activity of oridonin and arsenic trioxide on hepatocellular carcinoma cells. International Journal of Oncology, 40(1), 139–147.
Herrerias, T., et al. (2010). Effects of natural flavones on membrane properties and citotoxicity of HeLa cells. Revista Brasileira de Farmacognosia, 20(3), 403–408.
Ou, H. C., et al. (2010). Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicology and Applied Pharmacology, 248(2), 134–143.
Chen, X., et al. (2011). No protective effect of curcumin on hydrogen peroxide-induced cytotoxicity in HepG2 cells. Pharmacological Reports, 63(3), 724–732.
Wijesekara, I., et al. (2014). Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma HeLa cells. Microbiological Research, 169(4), 255–261.
Xie, Q. C., & Yang, Y. P. (2014). Anti-proliferative of physcion 8-O-beta-glucopyranoside isolated from Rumex japonicus Houtt. on A549 cell lines via inducing apoptosis and cell cycle arrest. BMC Complementary and Alternative Medicine, 14, 377.
Li, S., et al. (2010). Icariin, a natural flavonol glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via a ROS/JNK-dependent mitochondrial pathway. Cancer Letters, 298(2), 222–230.
Yuan, L., et al. (2012). Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicology and Applied Pharmacology, 265(1), 83–92.
Raza, H., John, A., & Benedict, S. (2011). Acetylsalicylic acid-induced oxidative stress, cell cycle arrest, apoptosis and mitochondrial dysfunction in human hepatoma HepG2 cells. European Journal of Pharmacology, 668(1–2), 15–24.
Lee, J. O., et al. (2014). Rhus verniciflua extract modulates survival of MCF-7 breast cancer cells through the modulation of AMPK-pathway. Biological &/and Pharmaceutical Bulletin, 37(5), 794–801.
Hardie, D. G., & Alessi, D. R. (2013). LKB1 and AMPK and the cancer-metabolism link-ten years after. BMC Biology, 11, 36.
Imamura, K., et al. (2001). Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochemical and Biophysical Research Communications, 287(2), 562–567.
Jones, R. G., et al. (2005). AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Molecular Cell, 18(3), 283–293.
Gwinn, D. M., et al. (2008). AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular Cell, 30(2), 214–226.
Inoki, K., Zhu, T., & Guan, K. L. (2003). TSC2 mediates cellular energy response to control cell growth and survival. Cell, 115(5), 577–590.
Hardie, D. G. (2004). The AMP-activated protein kinase pathway—new players upstream and downstream. Journal of Cell Science, 117(Pt 23), 5479–5487.
Hardie, D. G., & Sakamoto, K. (2006). AMPK: A key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda), 21, 48–60.
Garcia-Gil, M., et al. (2003). 5′-aminoimidazole-4-carboxamide riboside induces apoptosis in human neuroblastoma cells. Neuroscience, 117(4), 811–820.
Kefas, B. A., et al. (2004). Metformin-induced stimulation of AMP-activated protein kinase in beta-cells impairs their glucose responsiveness and can lead to apoptosis. Biochemical Pharmacology, 68(3), 409–416.
Acknowledgments
This study was supported by Application Foundation Project of Science & Technology Agency of Sichuan Province (14JC0804).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiong, Y., Ren, L., Wang, Z. et al. Anti-proliferative Effect of Physcion on Human Gastric Cell Line via Inducing ROS-Dependent Apoptosis. Cell Biochem Biophys 73, 537–543 (2015). https://doi.org/10.1007/s12013-015-0674-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0674-9