Abstract
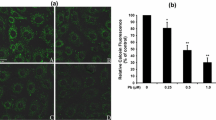
Lead and mercury are the ubiquitous heavy metals triggering toxicity and initiating apoptosis in cells. Though the toxic effects of heavy metals on various organs are known, there is a paucity of information on the mechanisms that instigate the current study. A plausible role of phospholipid scramblase 3 (PLSCR3) in Pb2+ and Hg2+ induced apoptosis was investigated with human embryonic kidney (HEK 293) cells. After 12 h of exposure, ~30–40% of the cells were in the early stage of apoptosis with increased reactive oxygen species (ROS), decreased mitochondrial membrane potential, and increased intracellular calcium levels. Also, ~20% of the cardiolipin localized within the inner mitochondrial membrane was translocated to the outer mitochondrial membrane along with the mobilization of truncated Bid (t-Bid) to the mitochondria and cytochrome c from the mitochondria. The endogenous expression levels of PLSCR3, caspase 8, and caspase 3 were upregulated in Pb2+ and Hg2+ induced apoptosis. The activation and upregulation of PLSCR3 mediate CL translocation playing a potential role in initiating the heavy metal-induced apoptosis. Therefore, PLSCR3 could be the linker between mitochondria and heavy metal apoptosis.








Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Järup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68(1), 167–182. https://doi.org/10.1093/bmb/ldg032.
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B. B., & Beeregowda, K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicology, 7(2), 60–72. https://doi.org/10.2478/intox-2014-0009.
Kim, J.-J., Kim, Y.-S., & Kumar, V. (2019). Heavy metal toxicity: an update of chelating therapeutic strategies. Journal of Trace Elements in Medicine and Biology, 54, 226–231. https://doi.org/10.1016/j.jtemb.2019.05.003.
Castellino, N., & Aloj, S. (1969). Intracellular distribution of lead in the liver and kidney of the rat. Occupational and Environmental Medicine, 26(2), 139–143. https://doi.org/10.1136/oem.26.2.139.
Pulido, M. D., & Parrish, A. R. (2003). Metal-induced apoptosis: mechanisms. Mutation Research, 533(1–2), 227–241.
Wu, X., Cobbina, S. J., Mao, G., Xu, H., Zhang, Z., & Yang, L. (2016). A review of toxicity and mechanisms of individual and mixtures of heavy metals in the environment. Environmental Science and Pollution Research, 23(9), 8244–8259. https://doi.org/10.1007/s11356-016-6333-x.
Pearce, J. M. S. (2007). Burton’s line in lead poisoning. European Neurology, 57(2), 118–119. https://doi.org/10.1159/000098100.
Flora, G., Gupta, D., & Tiwari, A. (2012). Toxicity of lead: a review with recent updates. Interdisciplinary Toxicology, 5(2), 47–58. https://doi.org/10.2478/v10102-012-0009-2.
Garza, A., Vega, R., & Soto, E. (2006). Cellular mechanisms of lead neurotoxicity. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 12(3), RA57–RA65.
Schirrmacher, K., Wiemann, M., Bingmann, D., & Büsselberg, D. (1998). Effects of lead, mercury, and methyl mercury on gap junctions and [Ca2+]i in bone cells. Calcified Tissue International, 63(2), 134–139. https://doi.org/10.1007/s002239900503.
Shenker, B. J., Guo, T. L., & Shapiro, I. M. (2000). Mercury-induced apoptosis in human lymphoid cells: evidence that the apoptotic pathway is mercurial species dependent. Environmental Research, 84(2), 89–99. https://doi.org/10.1006/enrs.2000.4078.
Farina, M., Rocha, J. B. T., & Aschner, M. (2011). Mechanisms of methylmercury-induced neurotoxicity: evidence from experimental studies. Life Sciences, 89(15–16), 555–563. https://doi.org/10.1016/j.lfs.2011.05.019.
Cobbina, S. J., Chen, Y., Zhou, Z., Wu, X., Feng, W., Wang, W., & Yang, L. (2015). Low concentration toxic metal mixture interactions: effects on essential and non-essential metals in brain, liver, and kidneys of mice on sub-chronic exposure. Chemosphere, 132, 79–86. https://doi.org/10.1016/j.chemosphere.2015.03.013.
Cobbina, S. J., Chen, Y., Zhou, Z., Wu, X., Zhao, T., Zhang, Z., & Yang, L. (2015). Toxicity assessment due to sub-chronic exposure to individual and mixtures of four toxic heavy metals. Journal of Hazardous Materials, 294, 109–120. https://doi.org/10.1016/j.jhazmat.2015.03.057.
Renu, K., Chakraborty, R., Myakala, H., Koti, R., Famurewa, A. C., Madhyastha, H., & Valsala Gopalakrishnan, A. (2021). Molecular mechanism of heavy metals (lead, chromium, arsenic, mercury, nickel and cadmium)—induced hepatotoxicity—a review. Chemosphere, 271, 129735. https://doi.org/10.1016/j.chemosphere.2021.129735.
Ferri, K. F., & Kroemer, G. (2001). Organelle-specific initiation of cell death pathways. Nature Cell Biology, 3(11), E255–E263. https://doi.org/10.1038/ncb1101-e255.
Estaquier, J., Vallette, F., Vayssiere, J.-L., & Mignotte, B. (2012). The mitochondrial pathways of apoptosis. In R. Scatena, P. Bottoni, & B. Giardina (Eds.), Advances in mitochondrial medicine (942, 157–183). Dordrecht: Springer Netherlands. https://doi.org/10.1007/978-94-007-2869-1_7
Nieminen, A. L., Gores, G. J., Dawson, T. L., Herman, B., & Lemasters, J. J. (1990). Toxic injury from mercuric chloride in rat hepatocytes. The Journal of Biological Chemistry, 265(4), 2399–2408.
Belyaeva, E. A., Glazunov, V. V., & Korotkov, S. M. (2004). Cd2+ -promoted mitochondrial permeability transition: a comparison with other heavy metals. Acta Biochimica Polonica, 51(2), 545–551. doi: 035001545.
Belyaeva, E. A., Sokolova, T. V., Emelyanova, L. V., & Zakharova, I. O. (2012). Mitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copper. The Scientific World Journal, 2012, 1–14. https://doi.org/10.1100/2012/136063.
Belyaeva, E. A., Dymkowska, D., Więckowski, M. R., & Wojtczak, L. (2008). Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicology and Applied Pharmacology, 231(1), 34–42. https://doi.org/10.1016/j.taap.2008.03.017.
Li, M. (2003). Cadmium directly induced the opening of membrane permeability pore of mitochondria which possibly involved in cadmium-triggered apoptosis. Toxicology, 194(1–2), 19–33. https://doi.org/10.1016/S0300-483X(03)00327-5.
Meyer, J. N., Leung, M. C. K., Rooney, J. P., Sendoel, A., Hengartner, M. O., Kisby, G. E., & Bess, A. S. (2013). Mitochondria as a target of environmental toxicants. Toxicological Sciences, 134(1), 1–17. https://doi.org/10.1093/toxsci/kft102.
Chen, F., Vallyathan, V., Castranova, V., & Shi, X. (2001). Cell apoptosis induced by carcinogenic metals. Molecular and Cellular Biochemistry, 222(1–2), 183–188.
Petit, P. X., Susin, S. A., Zamzami, N., Mignotte, B., & Kroemer, G. (1996). Mitochondria and programmed cell death: back to the future. FEBS letters, 396(1), 7–13.
Farina, M., Avila, D. S., da Rocha, J. B. T., & Aschner, M. (2013). Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochemistry International, 62(5), 575–594. https://doi.org/10.1016/j.neuint.2012.12.006.
He, L., Perkins, G. A., Poblenz, A. T., Harris, J. B., Hung, M., Ellisman, M. H., & Fox, D. A. (2003). Bcl-xL overexpression blocks bax-mediated mitochondrial contact site formation and apoptosis in rod photoreceptors of lead-exposed mice. Proceedings of the National Academy of Sciences, 100(3), 1022–1027. https://doi.org/10.1073/pnas.0333594100.
He, L., Poblenz, A. T., Medrano, C. J., & Fox, D. A. (2000). Lead and calcium produce rod photoreceptor cell apoptosis by opening the mitochondrial permeability transition pore. Journal of Biological Chemistry, 275(16), 12175–12184. https://doi.org/10.1074/jbc.275.16.12175.
Kim, S. H., & Sharma, R. P. (2004). Mercury-induced apoptosis and necrosis in murine macrophages: role of calcium-induced reactive oxygen species and p38 mitogen-activated protein kinase signaling. Toxicology and Applied Pharmacology, 196(1), 47–57. https://doi.org/10.1016/j.taap.2003.11.020.
Liu, J., Dai, Q., Chen, J., Durrant, D., Freeman, A., Liu, T., & Lee, R. M. (2003). Phospholipid scramblase 3 controls mitochondrial structure, function, and apoptotic response. Molecular cancer research: MCR, 1(12), 892–902.
Dudek, J. (2017). Role of cardiolipin in mitochondrial signaling pathways. Frontiers in Cell and Developmental Biology, 5, 90. https://doi.org/10.3389/fcell.2017.00090.
Garcia Fernandez, M., Troiano, L., Moretti, L., Nasi, M., Pinti, M., Salvioli, S., & Cossarizza, A. (2002). Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth & Differentiation: The Molecular Biology Journal of the American Association for Cancer Research, 13(9), 449–455.
Ndebele, K., Gona, P., Jin, T.-G., Benhaga, N., Chalah, A., Degli-Esposti, M., & Khosravi-Far, R. (2008). Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) induced mitochondrial pathway to apoptosis and caspase activation is potentiated by phospholipid scramblase-3. Apoptosis, 13(7), 845–856. https://doi.org/10.1007/s10495-008-0219-4.
Liu, J., Epand, R. F., Durrant, D., Grossman, D., Chi, N., Epand, R. M., & Lee, R. M. (2008). Role of phospholipid scramblase 3 in the regulation of tumor necrosis factor-α-induced apoptosis†. Biochemistry, 47(15), 4518–4529. https://doi.org/10.1021/bi701962c.
Liu, J., Chen, J., Dai, Q., & Lee, R. M. (2003). Phospholipid scramblase 3 is the mitochondrial target of protein kinase C delta-induced apoptosis. Cancer Research, 63(6), 1153–1156.
Palanirajan, S. K., & Gummadi, S. N. (2020). Heavy-metals-mediated phospholipids scrambling by human phospholipid scramblase 3: a probable role in mitochondrial apoptosis. Chemical Research in Toxicology, 33(2), 553–564. https://doi.org/10.1021/acs.chemrestox.9b00406.
Garcia Fernandez, M., Troiano, L., Moretti, L., Pedrazzi, J., Salvioli, S., Castilla-Cortazar, I., & Cossarizza, A. (2000). Changes in intramitochondrial cardiolipin distribution in apoptosis-resistant HCW-2 cells, derived from the human promyelocytic leukemia HL-60. FEBS Letters, 478(3), 290–294. https://doi.org/10.1016/S0014-5793(00)01861-5.
Dimauro, I., Pearson, T., Caporossi, D., & Jackson, M. J. (2012). A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Research Notes, 5(1), 513 https://doi.org/10.1186/1756-0500-5-513.
Stohs, S. (1995). Oxidative mechanisms in the toxicity of metal ions. Free Radical Biology and Medicine, 18(2), 321–336. https://doi.org/10.1016/0891-5849(94)00159-H.
Gstraunthaler, G., Pfaller, W., & Kotanko, P. (1983). Glutathione depletion and in vitro lipid peroxidation in mercury or maleate induced acute renal failure. Biochemical Pharmacology, 32(19), 2969–2972. https://doi.org/10.1016/0006-2952(83)90404-5.
Fukino, H., Hirai, M., Hsueh, Y. M., & Yamane, Y. (1984). Effect of zinc pretreatment on mercuric chloride-induced lipid peroxidation in the rat kidney. Toxicology and Applied Pharmacology, 73(3), 395–401. https://doi.org/10.1016/0041-008X(84)90091-7.
Matović, V., Buha, A., Ðukić-Ćosić, D., & Bulat, Z. (2015). Insight into the oxidative stress induced by lead and/or cadmium in blood, liver and kidneys. Food and Chemical Toxicology, 78, 130–140. https://doi.org/10.1016/j.fct.2015.02.011.
Ahyayauch, H., García-Arribas, A. B., Sot, J., González-Ramírez, E. J., Busto, J. V., Monasterio, B. G., & Goñi, F. M. (2018). Pb(II) induces scramblase activation and ceramide-domain generation in red blood cells. Scientific Reports, 8(1), 7456. https://doi.org/10.1038/s41598-018-25905-8.
Tan, X., Tang, C., Castoldi, A. F., Manzo, L., & Costa, L. G. (1993). Effects of inorganic and organic mercury on intracellular calcium levels in rat t lymphocytes. Journal of Toxicology and Environmental Health, 38(2), 159–170. https://doi.org/10.1080/15287399309531709.
Florea, A.-M., & Büsselberg, D. (2005). Toxic effects of metals: modulation of intracellular calcium homeostasis. Materialwissenschaft und Werkstofftechnik, 36(12), 757–760. https://doi.org/10.1002/mawe.200500960.
Fox, D. A., He, L., Poblenz, A. T., Medrano, C. J., Blocker, Y. S., & Srivastava, D. (1998). Lead-induced alterations in retinal cGMP phosphodiesterase trigger calcium overload, mitochondrial dysfunction and rod photoreceptor apoptosis. Toxicology Letters, 102–103, 359–361. https://doi.org/10.1016/S0378-4274(98)00232-X.
Wilson, B. A., Ramanathan, A., & Lopez, C. F. (2019). Cardiolipin-dependent properties of model mitochondrial membranes from molecular simulations. Biophysical Journal, 117(3), 429–444. https://doi.org/10.1016/j.bpj.2019.06.023.
Paradies, G., Paradies, V., Ruggiero, F. M., & Petrosillo, G. (2019). Role of cardiolipin in mitochondrial function and dynamics in health and disease: molecular and pharmacological aspects. Cells, 8(7), 728. https://doi.org/10.3390/cells8070728.
Van, Q., Liu, J., Lu, B., Feingold, K. R., Shi, Y., Lee, R. M., & Hatch, G. M. (2007). Phospholipid scramblase-3 regulates cardiolipin de novo biosynthesis and its resynthesis in growing HeLa cells. Biochemical Journal, 401(1), 103–109. https://doi.org/10.1042/BJ20060373.
Rouillard, A. D., Gundersen, G. W., Fernandez, N. F., Wang, Z., Monteiro, C. D., McDermott, M. G, & Ma’ayan, A. (2016). The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database, 2016, baw100. https://doi.org/10.1093/database/baw100.
Uhlen, M., Fagerberg, L., Hallstrom, B. M., Lindskog, C., Oksvold, P., Mardinoglu, A., & Ponten, F. (2015). Tissue-based map of the human proteome. Science, 347(6220), 1260419–1260419. https://doi.org/10.1126/science.1260419.
Wiedmer, T., Zhou, Q., Kwoh, D. Y., & Sims, P. J. (2000). Identification of three new members of the phospholipid scramblase gene family. Biochimica et Biophysica Acta, 1467(1), 244–253.
Nabergoj, D., Vrbek, S., Zidar, N., Tomašić, T., Kikelj, D., Mašič, L. P., & Muller, C. D. (2016). Synthetic analogues of marine alkaloid clathrodin differently induce phosphatidylserine exposure in monocytic cancer cells then in cancer stem cell lines. MedChemComm, 7(8), 1546–1554. https://doi.org/10.1039/C6MD00163G.
Ghosh, S., Basu Ball, W., Madaris, T. R., Srikantan, S., Madesh, M., Mootha, V. K., & Gohil, V. M. (2020). An essential role for cardiolipin in the stability and function of the mitochondrial calcium uniporter. Proceedings of the National Academy of Sciences, 117(28), 16383–16390. https://doi.org/10.1073/pnas.2000640117.
Cheng, Y.-J., Yang, B.-C., Hsieh, W.-C., Huang, B.-M., & Liu, M.-Y. (2002). Enhancement of TNF-α expression does not trigger apoptosis upon exposure of glial cells to lead and lipopolysaccharide. Toxicology, 178(3), 183–191. https://doi.org/10.1016/S0300-483X(02)00225-1.
He, Y., Liu, J., Durrant, D., Yang, H.-S., Sweatman, T., Lothstein, L., & Lee, R. M. (2005). N -Benzyladriamycin-14-valerate (AD198) induces apoptosis through protein kinase C-δ–induced phosphorylation of phospholipid scramblase 3. Cancer Research, 65(21), 10016–10023. https://doi.org/10.1158/0008-5472.CAN-05-1688.
He, Y., Liu, J., Grossman, D., Durrant, D., Sweatman, T., Lothstein, L., & Lee, R. M. (2007). Phosphorylation of mitochondrial phospholipid scramblase 3 by protein kinase C-δ induces its activation and facilitates mitochondrial targeting of tBid. Journal of Cellular Biochemistry, 101(5), 1210–1221. https://doi.org/10.1002/jcb.21243.
Sandra, F., Degli Esposti, M., Ndebele, K., Gona, P., Knight, D., Rosenquist, M., & Khosravi-Far, R. (2005). Tumor necrosis factor–related apoptosis-inducing ligand alters mitochondrial membrane lipids. Cancer Research, 65(18), 8286–8297. https://doi.org/10.1158/0008-5472.CAN-04-1913.
Acknowledgements
Authors acknowledge the Indian Institute of Technology Madras for facilities. SKP wishes to thank the Ministry of Human Resource Development (MHRD), Government of India, and Indian Institute of Technology Madras for fellowship. The authors wish to thank Mr. Praveen Kumar for his support in FACS data acquisition. The authors thank Prof. Suresh Kumar Rayala and Prof. Mukesh Doble IITM, for their generous donation of the cell lines and Bid antibody.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SKP: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Visualization, Writing—original draft, review & editing. SNG: Conceptualization, Supervision, Resources, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Palanirajan, S.K., Gummadi, S.N. Phospholipid scramblase 3: a latent mediator connecting mitochondria and heavy metal apoptosis. Cell Biochem Biophys 81, 443–458 (2023). https://doi.org/10.1007/s12013-023-01145-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-023-01145-0