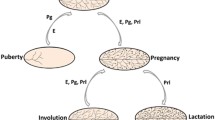
Abstract
The isolation and characterisation of mammary stem cells is an important step towards elucidating the hierarchy of epithelial cell development in the mammary gland and identifying cells that are targets of breast carcinogenesis. Mammary stem cells have recently been prospectively isolated through the identification of specific cell surface markers and in vivo transplantation into cleared fat pads. These cells were demonstrated to reconstitute an entire mammary gland comprising all mature epithelial cell types and to be capable of self-renewal on serial transplantation, thus possessing the defining features of stem cells. Notably, mouse mammary stem cells were found to share the hallmark properties of the basal subtype of breast cancer. This review will summarize the strategy used in the identification of mouse mammary stem cells and their characterisation.


Similar content being viewed by others
References
DeOme, K. B., Faulkin, L. J., Jr., Bern, H. A., & Blair, P. B. (1959). Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Research, 19, 515–520.
Smith, G. H., & Medina, D. (1988). A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. Journal of Cell Science, 90(Pt 1), 173–183.
Daniel, C. W., De Ome, K. B., Young, J. T., Blair, P. B., & Faulkin, L. J., Jr. (1968). The in vivo life span of normal and preneoplastic mouse mammary glands: A serial transplantation study. Proceedings of the National Academy of Sciences of the United States of America, 61, 53–60.
Daniel, C. W., & Young, L. J. (1971). Influence of cell division on an aging process. Life span of mouse mammary epithelium during serial propagation in vivo. Experimental Cell Research, 65, 27–32.
Kordon, E. C., & Smith, G. H. (1998). An entire functional mammary gland may comprise the progeny from a single cell. Development, 125, 1921–1930.
Chepko, G., & Smith, G. H. (1997). Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue & Cell, 29, 239–253.
Welm, B. E., Tepera, S. B., Venezia, T., Graubert, T. A., Rosen, J. M., & Goodell, M. A. (2002). Sca-1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Developmental Biology, 245, 42–56.
Alvi, A. J., Clayton, H., Joshi, C., Enver, T., Ashworth, A., Vivanco, M. M., et al. (2003). Functional and molecular characterisation of mammary side population cells. Breast Cancer Research, 5, R1–8.
Stingl, J., Eaves, C. J., Zandieh, I., & Emerman, J. T. (2001). Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Research and Treatment, 67, 93–109.
Gudjonsson, T., Villadsen, R., Nielsen, H. L., Ronnov-Jessen, L., Bissell, M. J., & Petersen, O. W. (2002). Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes and Development, 16, 693–706.
Dontu, G., Abdallah, W. M., Foley, J. M., Jackson, K. W., Clarke, M. F., Kawamura, M. J., et al. (2003). In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes and Development, 17, 1253–1270.
Weissman, I. L. (2000). Stem cells: Units of development, units of regeneration, and units in evolution. Cell, 100, 157–168.
Dick, J. E. (2003). Stem cells: Self-renewal writ in blood. Nature, 423, 231–233.
Krause, D. S., Theise, N. D., Collector, M. I., Henegariu, O., Hwang, S., Gardner, R., et al. (2001). Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell, 105, 369–377.
Uchida, N., Dykstra, B., Lyons, K. J., Leung, F. Y., & Eaves, C. J. (2003). Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Experimental Hematology, 31, 1338–1347.
Matsuzaki, Y., Kinjo, K., Mulligan, R. C., & Okano, H. (2004). Unexpectedly efficient homing capacity of purified murine hematopoietic stem cells. Immunity, 20, 87–93.
Takano, H., Ema, H., Sudo, K., & Nakauchi, H. (2004). Asymmetric division and lineage commitment at the level of hematopoietic stem cells: Inference from differentiation in daughter cell and granddaughter cell pairs. Journal of Experimental Medicine, 199, 295–302.
Shackleton, M., Vaillant, F., Simpson, K. J., Stingl, J., Smyth, G. K., Asselin-Labat, M. L., et al. (2006). Generation of a functional mammary gland from a single stem cell. Nature, 439, 84–88.
Stingl, J., Eirew, P., Ricketson, I., Shackleton, M., Vaillant, F., Choi, D., et al., (2006). Purification and unique properties of mammary epithelial stem cells. Nature, 439, 993–997.
Uchida, N., & Weissman, I. L. (1992). Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin- Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. Journal of Experimental Medicine, 175, 175–184.
Bonnefoix, T., Bonnefoix, P., Verdiel, P., & Sotto, J. J. (1996). Fitting limiting dilution experiments with generalized linear models results in a test of the single-hit Poisson assumption. Journal of Immunological Methods, 194, 113–119.
Sleeman, K. E., Kendrick, H., Ashworth, A., Isacke, C. M., & Smalley, M. J. (2006). CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Research, 8, R7.
Smith, G. H. (1996). Experimental mammary epithelial morphogenesis in an in vivo model: evidence for distinct cellular progenitors of the ductal and lobular phenotype. Breast Cancer Research and Treatment, 39, 21–31.
Brisken, C., Park, S., Vass, T., Lydon, J. P., O'Malley, B. W., & Weinberg, R. A. (1998). A paracrine role for the epithelial progesterone receptor in mammary gland development. Proceedings of the National Academy of Sciences of the United States of America, 95, 5076–5081.
Boulanger, C. A., Wagner, K., U., & Smith, G. H. (2005). Parity-induced mouse mammary epithelial cells are pluripotent, self-renewing and sensitive to TGF-beta1 expression. Oncogene, 24, 552–560.
Wagner, K. U., Boulanger, C. A., Henry, M. D., Sgagias, M., Hennighausen, L., & Smith, G. H. (2002). An adjunct mammary epithelial cell population in parous females: Its role in functional adaptation and tissue renewal. Development, 129, 1377–1386.
Ito, C. Y., Li, C. Y., Bernstein, A., Dick, J. E., & Stanford, W. L. (2003). Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood, 101, 517–523.
Zhou, S., Schuetz, J. D., Bunting, K. D., Colapietro, A. M., Sampath, J., Morris, J. J., et al. (2001). The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nature Medicine, 7, 1028–1034.
Goodell, M. A., Brose, K., Paradis, G., Conner, A. S., & Mulligan, R. C. (1996). Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. Journal of Experimental Medicine, 183, 1797–1806.
Li, Y., Welm, B., Podsypanina, K., Huang, S., Chamorro, M., Zhang, X., et al. (2003). Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 15853–15858.
Liu, B. Y., McDermott, S. P., Khwaja, S. S., & Alexander, C. M. (2004). The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proceedings of the National Academy of Sciences of the United States of America, 101, 4158–4163.
Triel, C., Vestergaard, M. E., Bolund, L., Jensen, T. G., & Jensen, U. B. (2004). Side population cells in human and mouse epidermis lack stem cell characteristics. Experimental Cell Research, 295, 79–90.
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L., & Fuchs, E. (2004). Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell, 118, 635–648.
Kubota, H., Avarbock, M. R., & Brinster, R. L. (2003). Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 6487–6492.
Smith, G. H. (2005). Label-retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development, 132, 681–687.
Clarke, R. B., Spence, K., Anderson, E., Howell, A., Okano, H., & Potten, C. S. (2005). A putative human breast stem cell population is enriched for steroid receptor-positive cells. Developmental Biology, 277, 443–456.
Cheng T., Rodrigues, N., Shen, H., Yang, Y., Dombkowski, D., Sykes, M., et al. (2000). Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science, 287, 1804–1808.
Topley, G. I., Okuyama, R., Gonzales, J. G., Conti, C., & Dotto, G. P. (1999). p21(WAF1/Cip1) functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential. Proceedings of the National Academy of Sciences of the United States of America, 96, 9089–9094.
Potten, C. S., Booth, C., Tudor, G. L., Booth, D., Brady, G., Hurley, P., et al. (2003). Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation, 71, 28–41.
Okano, H., Imai, T., & Okabe, M. (2002). Musashi: A translational regulator of cell fate. Journal of Cell Science, 115, 1355–1359.
Smith, G. H., Mehrel, T., & Roop, D. R. (1990). Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth & Differentiation, 1, 161–170.
Cianfrocca, M., & Goldstein, L. J. (2004). Prognostic and predictive factors in early-stage breast cancer. Oncologist, 9, 606–616.
Esteva, F. J., & Hortobagyi, G. N. (2004). Prognostic molecular markers in early breast cancer. Breast Cancer Research, 6, 109–118.
Anderson, E. (2002). The role of oestrogen and progesterone receptors in human mammary development and tumorigenesis. Breast Cancer Research, 4, 197–201.
Asselin-Labat, M. L., Shackleton, M., Stingl, J., Vaillant, F., Forrest, N. C., Eaves, C. J., et al. (2006). Steroid hormone receptor status of mouse mammary stem cells. Journal of the National Cancer Institute, 98, 1011–1014.
Clarke, R. B., Howell, A., Potten, C. S., & Anderson, E. (1997). Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Research, 57, 4987–4991.
Russo, J., Ao, X., Grill, C., & Russo, I. H. (1999). Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Research and Treatment, 53, 217–227.
Booth, B. W., & Smith, G. H. (2006). Estrogen receptor-alpha and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Research, 8, R49.
Dontu, G., El-Ashry, D., & Wicha, M. S. (2004). Breast cancer, stem/progenitor cells and the estrogen receptor. Trends in Endocrinology and Metabolism, 15, 193–197.
Clarke, R. B. (2005). Isolation and characterization of human mammary stem cells. Cell Proliferation, 38, 375–386.
Asselin-Labat, M. L., Sutherland, K. D., Barker, H., Thomas, R., Shackleton, M., Forrest, N. C., et al. (2007). Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nature Cell Biology, 9, 201–209.
Sleeman, K. E., Kendrick, H., Robertson, D., Isacke, C. M., Ashworth, A., & Smalley, M. J. (2007). Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. Journal of Cell Biology, 176, 19–26.
Watt, F. M., & Hogan, B. L. (2000). Out of Eden: stem cells and their niches. Science, 287, 1427–1430.
Taddei, I., Faraldo, M. M., Teuliere, J., Deugnier, M. A., Thiery, J. P., & Glukhova, M. A. (2003). Integrins in mammary gland development and differentiation of mammary epithelium. Journal of Mammary Gland Biology and Neoplasia, 8, 383–394.
Shaw, L. M. (1999). Integrin function in breast carcinoma progression. Journal of Mammary Gland Biology and Neoplasia, 4, 367–376.
White, J. M. (2003). ADAMs: Modulators of cell–cell and cell–matrix interactions. Current Opinion in Cell Biology, 15, 598–606
Klinowska, T. C., Soriano, J. V., Edwards, G. M., Oliver, J. M., Valentijn, A. J., Montesano, R., et al. (1999). Laminin and beta1 integrins are crucial for normal mammary gland development in the mouse. Developmental Biology, 215, 13–32.
Naylor, M. J., Li, N., Cheung, J., Lowe, E. T., Lambert, E., Marlow, R., et al. (2005). Ablation of b1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. Journal of Cell Biology, 171, 717–728.
Li, N., Zhang, Y., Naylor, M. J., Schatzmann, F., Maurer, F., Wintermantel, T., Schuetz, et al. (2005). Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO Journal, 24, 1942–1953.
Klinowska, T. C., Alexander, C. M., Georges-Labouesse, E., Van der Neut, R., Kreidberg, J. A., Jones, C. J., et al. (2001). Epithelial development and differentiation in the mammary gland is not dependent on a3 or a6 integrin subunits. Developmental Biology, 233, 449–467.
Ramalho-Santos, M., Yoon, S., Matsuzaki, Y., Mulligan, R. C., & Melton, D. A. (2002). “Stemness”: Transcriptional profiling of embryonic and adult stem cells. Science, 298, 597–600.
Ivanova, N. B., Dimos, J. T., Schaniel, C., Hackney, J. A., Moore, K. A., & Lemischka, I. R. (2002). A stem cell molecular signature. Science, 298, 601–604.
Wagers, A. J., Allsopp, R. C., & Weissman, I. L. (2002). Changes in integrin expression are associated with altered homing properties of Lin(−/lo)Thy1.1(lo)Sca-1(+)c-kit(+) hematopoietic stem cells following mobilization by cyclophosphamide/granulocyte colony-stimulating factor. Experimental Hematology, 30, 176–185.
Campos, L. S., Leone, D. P., Relvas, J. B., Brakebusch, C., Fassler, R., Suter, U., et al. (2004). Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development, 131, 3433–3444.
Tani, H., Morris, R. J., & Kaur, P. (2000). Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proceedings of the National Academy of Sciences of the United States of America, 97, 10960–10965.
Jones, P. H., & Watt, F. M. (1993). Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell, 73, 713–724.
Shinohara, T., Avarbock, M. R., & Brinster, R. L. (1999). beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proceedings of the National Academy of Sciences of the United States of America, 96, 5504–5509.
Lin, E. Y., Jones, J. G., Li, P., Zhu, L., Whitney, K. D., Muller, W. J., et al. (2003). Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. American Journal of Pathology, 163, 2113–2126.
White, D. E., Kurpios, N. A., Zuo, D., Hassell, J. A., Blaess, S., Mueller, U., et al. (2004). Targeted disruption of b1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell, 6, 159–170.
Nielsen, T. O., Hsu, F. D., Jensen, K., Cheang, M., Karaca, G., Hu, Z., et al. (2004). Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clinical Cancer Research, 10, 5367–5374.
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 3983–3988.
Schabath, H., Runz, S., Joumaa, S., & Altevogt, P. (2006). CD24 affects CXCR4 function in pre-B lymphocytes and breast carcinoma cells. Journal of Cell Science, 119, 314–325.
Kuperwasser, C., Chavarria, T., Wu, M., Magrane, G., Gray, J. W., Carey, L., et al. (2004). Reconstruction of functionally normal and malignant human breast tissues in mice. Proceedings of the National Academy of Sciences of the United States of America, 101, 4966–4971.
Acknowledgments
Supported by the Victorian Breast Cancer Research Consortium Inc., Australia; the National Breast Cancer Foundation, Australia; and the National Health and Medical Research Council, Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaillant, F., Asselin-Labat, ML., Shackleton, M. et al. The Emerging Picture of the Mouse Mammary Stem Cell. Stem Cell Rev 3, 114–123 (2007). https://doi.org/10.1007/s12015-007-0018-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-007-0018-2