Abstract
The breakdown of self-tolerance of the immune response can lead to autoimmune conditions in which chronic inflammation induces tissue damage. Systemic lupus erythematosus (SLE) is a debilitating multisystemic autoimmune disorder with a high prevalence in women of childbearing age; however, SLE incidence, prevalence, and severity are strongly influenced by ethnicity. Although the mystery of autoimmune diseases remains unsolved, disturbance in the proportion and function of B cell subsets has a major role in SLE’s pathogenesis. Additionally, colocalizing hyperactive T helper cell subgroups within inflammatory niches are indispensable. Despite significant advances in standard treatments, nonspecific immunosuppression, the risk of serious infections, and resistance to conventional therapies in some cases have raised the urgent need for new treatment strategies. Without the need to suppress the immune system, mesenchymal stem cells (MSCs), as ‘‘smart” immune modulators, are able to control cellular and humoral auto-aggression responses by participating in precursor cell development. In lupus, due to autologous MSCs disorder, the ability of allogenic engrafted MSCs in tissue regeneration and resetting immune homeostasis with the provision of a new immunocyte repertoire has been considered simultaneously.
Graphical Abstract
In Brief
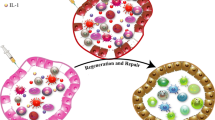
The bone marrow mesenchymal stem cells (BM-MSCs) lineage plays a critical role in maintaining the hematopoietic stem-cell microstructure and modulating immunocytes.
The impairment of BM-MSCs and their niche partially contribute to the pathogenesis of SLE-like diseases.
Allogenic MSC transplantation can reconstruct BM microstructure, possibly contributing to the recovery of immunocyte phenotype restoration of immune homeostasis.
In terms of future prospects of MSCs, artificially gained by ex vivo isolation and culture adaptation, the wide variety of potential mediators and mechanisms might be linked to the promotion of the immunomodulatory function of MSCs.



Similar content being viewed by others
Data Availability
There is no data to share because the article is a review.
Abbreviations
- Anti-dsDNA :
-
Anti-double stranded DNA
- BAFF :
-
B activating-cell factor
- Bcl-6 :
-
B-cell leukemia/lymphoma 6
- BM :
-
Bone marrow
- Breg :
-
Regulatory B cell
- CCL2 :
-
C-C motif chemokine ligand 2
- CCL18 :
-
C-C motif chemokine ligand 18
- CCR6:
-
Chemokine receptor 6
- CFU-Fs :
-
Fibroblast colony-forming units
- CCR2 :
-
C-C chemokine receptor type 2
- CXCR5 :
-
Chemokine Receptor 5
- CXCR4 :
-
Chemokine Receptor 4
- DC :
-
Dendritic Cell
- FAS/FASL :
-
Fas and Fas ligand
- GCs :
-
Germinal centers
- HGF :
-
Hepatocyte growth factor
- HLA :
-
Human leukocyte antigen
- HLA-G5 :
-
Human leukocyte antigen-G5
- ICAM-1 :
-
Intercellular adhesion molecule 1
- ICOS :
-
Inducible T cell co-stimulator
- IDO :
-
Indolamine 2, 3-dioxigenase
- IFN-γ :
-
Interferon-gamma
- IL :
-
Interleukin
- M2 :
-
Alternative macrophage
- MCP-1 :
-
Monocyte Chemotactic Protein-1
- MHC :
-
Major histocompatibility complex
- MSC :
-
Mesenchymal stem/stromal cell
- MV :
-
Membrane vesicle
- NO :
-
Nitric oxide
- NF-κB :
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- PCs :
-
Plasma cells
- PGE2 :
-
Prostaglandin E2
- SLE :
-
Systemic lupus erythematosus
- STAT3 :
-
Signal transducer and activator of transcription 3
- Tfh :
-
T follicular helper
- Tfr :
-
T follicular regulatory
- TGF-β :
-
Transforming growth factor-beta
- Th :
-
T helper cells
- TNF-α :
-
Tumor necrosis alpha-factor
- TRAF6/NF-κB :
-
TNF Receptor-associated factor 6
- Tr1 :
-
Type 1 regulatory T cells
- Treg :
-
T regulatory cell
- TSG-6 :
-
Tumour necrosis factor-Stimulated gene 6
- UV :
-
Ultraviolet
- UC :
-
Umbilical cord
- VCAM-1 :
-
Vascular cell adhesion molecule 1
References
Kapsogeorgou, E. K., & Tzioufas, A. G. (2016). Autoantibodies in autoimmune diseases: Clinical and critical evaluation. The Israel Medical Association Journal: IMAJ, 18(9), 519–524.
Rezaieyazdi, Z., et al. (2021). Outcomes of planned pregnancy in patients with systemic lupus erythematosus and their neonates. The Egyptian Rheumatologist, 43(2), 141–145.
Wang, L., Wang, F. S., & Gershwin, M. E. (2015). Human autoimmune diseases: A comprehensive update. Journal of internal medicine, 278(4), 369–395.
Ray, S., et al. (2012). Autoimmune disorders: An overview of molecular and cellular basis in today’s perspective. Journal of Clinical & Cellular Immunology, 10(003), 2215–2230.
Stojan, G., & Petri, M. (2018). Epidemiology of systemic lupus erythematosus: An update. Current opinion in rheumatology, 30(2), 144.
Rees, F., et al. (2017). The worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology, 56(11), 1945–1961.
Fava, A., & Petri, M. (2019). Systemic lupus erythematosus: Diagnosis and clinical management. Journal of autoimmunity, 96, 1–13.
Rezaieyazdi, Z., et al. (2018). No association between the risk of breast cancer and systemic lupus erythematosus: Evidence from a meta-analysis. Clinical rheumatology, 37(6), 1511–1519.
Barturen, G., & Alarcón-Riquelme, M. E. (2017). SLE redefined on the basis of molecular pathways. Best Practice & Research Clinical Rheumatology, 31(3), 291–305.
Toro-Domínguez, D., et al. (2018). Longitudinal stratification of gene expression reveals three SLE groups of disease activity progression. Arthritis & rheumatology (Hoboken, NJ), 70(12), 2025.
Zhan, Y., Guo, Y., & Lu, Q. (2016). Aberrant epigenetic regulation in the pathogenesis of systemic lupus erythematosus and its implication in precision medicine. Cytogenetic and genome research, 149(3), 141–155.
Katayama, S., et al. (2019). Delineating the healthy human skin UV response and early induction of interferon pathway in cutaneous lupus erythematosus. Journal of Investigative Dermatology.
Magro, R., et al. (2021). Vitamin D supplementation in systemic lupus erythematosus: Relationship to disease activity, fatigue and the interferon signature gene expression. BMC rheumatology, 5(1), 1–8.
Islam, M. A., et al. (2019). Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmunity reviews, 18(11), 102392.
Li, Y., & Wu, T. (2018). Proteomic approaches for novel systemic lupus erythematosus (SLE) drug discovery. Expert Opinion on Drug Discovery, 13(8), 765–777.
Choo, H.M.C., et al. (2019). Risk factors for cytomegalovirus disease in systemic lupus erythematosus (SLE): a systematic review. Advances in Rheumatology, 59.
Zen, M., et al. (2010). Hormones, immune response, and pregnancy in healthy women and SLE patients. Swiss Medical Weekly, 140(1314).
Hornsby, H. (1993). Psychological adjustment and systemic lupus erythematosus: a comparative study. University of Tasmania.
Taheri, M., et al. (2020). Exploring the role of non-coding RNAs in the pathophysiology of systemic lupus erythematosus. Biomolecules, 10(6), 937.
Geng, L., et al. (2020). Reduced let-7f in bone marrow-derived mesenchymal stem cells triggers Treg/Th17 imbalance in patients with systemic lupus erythematosus. Frontiers in immunology, 11, 233.
Geng, L., et al. (2019). MicroRNA-663 induces immune dysregulation by inhibiting TGF-β1 production in bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Cellular & molecular immunology, 16(3), 260–274.
Tan, W., et al. (2019). Let-7f-5p ameliorates inflammation by targeting NLRP3 in bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Biomedicine & Pharmacotherapy, 118, 109313.
Dong, C., et al. (2019). Circulating exosomes derived-miR-146a from systemic lupus erythematosus patients regulates senescence of mesenchymal stem cells. BioMed research international, 2019.
Li, D., et al. (2020). MiR-153-3p induces immune dysregulation by inhibiting PELI1 expression in umbilical cord-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Autoimmunity, 53(4), 201–209.
Moulton, V. R., et al. (2017). Pathogenesis of human systemic lupus erythematosus: A cellular perspective. Trends in molecular medicine, 23(7), 615–635.
Murphy, G., & Isenberg, D. A. (2019). New therapies for systemic lupus erythematosus—past imperfect, future tense. Nature Reviews Rheumatology, 15(7), 403–412.
Tsokos, G. C. (2020). Autoimmunity and organ damage in systemic lupus erythematosus. Nature immunology, 21(6), 605–614.
Kustiyah, A. R., et al. (2021). The normal ratio of Th17 and Th1 post-mesenchymal stem cells coculture with PBMCs of systemic lupus erythematosus patients. Open Access Macedonian Journal of Medical Sciences, 9(A), 169–176.
Cheng, R.-J., et al. (2019). Mesenchymal stem cells: allogeneic MSC may be immunosuppressive but autologous MSC are dysfunctional in lupus patients. Frontiers in cell and developmental biology, 285.
Marion, T. N., Postlethwaite, A. E. (2014). Chance, genetics, and the heterogeneity of disease and pathogenesis in systemic lupus erythematosus. In Seminars in immunopathology. Springer.
Anders, H.-J., et al. (2020). Lupus nephritis. Nature reviews Disease primers, 6(1), 1–25.
Tjwa, M., et al. (2003). VEGF and PlGF: Two pleiotropic growth factors with distinct roles in development and homeostasis. Cell and tissue research, 314(1), 5–14.
Friedenstein, A., Chailakhjan, R., & Lalykina, K. (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Proliferation, 3(4), 393–403.
Caplan, A. I. (2017). Mesenchymal stem cells: Time to change the name! Stem cells translational medicine, 6(6), 1445–1451.
Meirelles, Ld. S., Chagastelles, P. C., & Nardi, N. B. (2006). Mesenchymal stem cells reside in virtually all post-natal organs and tissues. Journal of cell science, 119(11), 2204–2213.
Kassem, M., Kristiansen, M., & Abdallah, B. M. (2004). Mesenchymal stem cells: Cell biology and potential use in therapy. Basic & clinical pharmacology & toxicology, 95(5), 209–214.
Christodoulou, I., et al. (2018). Mesenchymal stem cells in preclinical cancer cytotherapy: A systematic review. Stem Cell Research & Therapy, 9(1), 1–38.
Takayama, Y., Kusamori, K., & Nishikawa, M. (2021). Mesenchymal stem/stromal cells as next-generation drug delivery vehicles for cancer therapeutics. Expert opinion on drug delivery, 18(11), 1627–1642.
Pittenger, M. F., et al. (1999). Multilineage potential of adult human mesenchymal stem cells. Science, 284(5411), 143–147.
Formigli, L., et al. (2012). Dermal matrix scaffold engineered with adult mesenchymal stem cells and platelet-rich plasma as a potential tool for tissue repair and regeneration. Journal of tissue engineering and regenerative medicine, 6(2), 125–134.
De Pieri, A., Rochev, Y., & Zeugolis, D. I. (2021). Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. NPJ Regenerative Medicine, 6(1), 1–15.
Salem, H. K., & Thiemermann, C. (2010). Mesenchymal stromal cells: Current understanding and clinical status. Stem cells, 28(3), 585–596.
Stagg, J. (2007). Immune regulation by mesenchymal stem cells: Two sides to the coin. Tissue Antigens, 69(1), 1–9.
Wahl, E. A., et al. (2015). In vitro evaluation of scaffolds for the delivery of mesenchymal stem cells to wounds. BioMed Research International, 2015.
Wang, L., et al. (2013). Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: Safety and efficacy. Stem cells and development, 22(24), 3192–3202.
Kolf, C. M., Cho, E., & Tuan, R. S. (2007). Mesenchymal stromal cells: Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis research & therapy, 9(1), 1–10.
Yousefi, F., et al. (2019). Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life sciences, 221, 99–108.
Yousefi, F., et al. (2019). Immunoregulatory, proliferative and anti-oxidant effects of nanocurcuminoids on adipose-derived mesenchymal stem cells. EXCLI journal, 18, 405.
Yousefi, F., et al. (2019). Various strategies to improve efficacy of stem cell transplantation in multiple sclerosis: Focus on mesenchymal stem cells and neuroprotection. Journal of Neuroimmunology, 328, 20–34.
Shamili, F. H., et al. (2018). Exosomes derived from TRAIL-engineered mesenchymal stem cells with effective anti-tumor activity in a mouse melanoma model. International Journal of Pharmaceutics, 549(1–2), 218–229.
Zhu, Y., & Feng, X. (2018). Genetic contribution to mesenchymal stem cell dysfunction in systemic lupus erythematosus. Stem Cell Research & Therapy, 9(1), 1–6.
Lim, J. E., & Son, Y. (2017). Endogenous stem cells in homeostasis and aging. Tissue Engineering and Regenerative Medicine, 14(6), 679–698.
Bloor, A. J., et al. (2020). Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: A phase I, multicenter, open-label, dose-escalation study. Nature Medicine, 26(11), 1720–1725.
Kuçi, Z., et al. (2016). Mesenchymal stromal cells from pooled mononuclear cells of multiple bone marrow donors as rescue therapy in pediatric severe steroid-refractory graft-versus-host disease: A multicenter survey. Haematologica, 101(8), 985.
Jahanian, M., et al. (2021). Evaluation of Acellular Dermal Matrix (ADM) as a scaffold for adipose-derived stem cell transfer in the rat model. World Journal of Plastic Surgery, 10(2), 67.
Douek, D. C., et al. (2000). Assessment of thymic output in adults after haematopoietic stemcell transplantation and prediction of T-cell reconstitution. The Lancet, 355(9218), 1875–1881.
Luque-Campos, N., et al. (2019). Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Frontiers in Immunology, 10.
Le Blanc, K., et al. (2004). Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scandinavian Journal of Immunology, 60(3), 307–315.
Guan, J., et al. (2015). Mesenchymal stem cell modulates T follicular helper cell to induce immunotolerance of Islet Allograft. Transplantation Proceedings, 47(6), 2050–2056.
Kim, Y. U., et al. (2015). Regulation of autoimmune germinal center reactions in lupus-prone BXD2 mice by follicular helper T cells. PLoS ONE, 10(3), e0120294.
Lu, X., et al. (2019). The effects of human umbilical cord-derived mesenchymal stem cell transplantation on endometrial receptivity are associated with Th1/Th2 balance change and uNK cell expression of uterine in autoimmune premature ovarian failure mice. Stem cell research & therapy, 10(1), 1–11.
Choi, E. W., et al. (2011). Transplantation of CTLA4Ig gene-transduced adipose tissue-derived mesenchymal stem cells reduces inflammatory immune response and improves Th1/Th2 balance in experimental autoimmune thyroiditis. The journal of gene medicine, 13(1), 3–16.
Collins, E., & Gilkeson, G. (2013). Hematopoetic and mesenchymal stem cell transplantation in the treatment of refractory systemic lupus erythematosus—Where are we now? Clinical immunology, 148(3), 328–334.
Chang, J.-W., et al. (2011). Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell transplantation, 20(2), 245–258.
Anolik, J. H., et al. (2007). Delayed memory B cell recovery in peripheral blood and lymphoid tissue in systemic lupus erythematosus after B cell depletion therapy. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 56(9), 3044–3056.
Ma, X., et al. (2013). Allogenic mesenchymal stem cell transplantation ameliorates nephritis in lupus mice via inhibition of B-cell activation. Cell transplantation, 22(12), 2279–2290.
Xiao, Y., et al. (2013). Efficacy and safety of mesenchymal stromal cell treatment from related donors for patients with refractory aplastic anemia. Cytotherapy, 15(7), 760–766.
Lohan, P., et al. (2018). Third-party allogeneic mesenchymal stromal cells prevent rejection in a pre-sensitized high-risk model of corneal transplantation. Frontiers in immunology, 9, 2666.
Yang, J., et al. (2009). Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis & Rheumatism, 60(5), 1472–1483.
Li, X., et al. (2013). Mesenchymal SCT ameliorates refractory cytopenia in patients with systemic lupus erythematosus. Bone marrow transplantation, 48(4), 544–550.
Yousefi, F., et al. (2021). Comparative assessment of immunomodulatory, proliferative, and antioxidant activities of crocin and crocetin on mesenchymal stem cells. Journal of Cellular Biochemistry, 122(1), 29–42.
Wang, M., et al. (2016). Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Scientific reports, 6(1), 1–13.
Young, S. A., et al. (2018). Mechanically resilient injectable scaffolds for intramuscular stem cell delivery and cytokine release. Biomaterials, 159, 146–160.
Braid, L. R., et al. (2018). Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy, 20(2), 232–244.
Jo, H., et al. (2021). Applications of mesenchymal stem cells in skin regeneration and rejuvenation. International Journal of Molecular Sciences, 22(5), 2410.
Sun, L., et al. (2010). Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis & Rheumatism, 62(8), 2467–2475.
Liang, J., et al. (2010). Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Annals of the rheumatic diseases, 69(8), 1423–1429.
Zonari, A., et al. (2015). Polyhydroxybutyrate-co-hydroxyvalerate structures loaded with adipose stem cells promote skin healing with reduced scarring. Acta Biomaterialia, 17, 170–181.
Ra, J. C., et al. (2011). Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. Journal of translational medicine, 9(1), 1–11.
Choi, E. W., et al. (2016). Mesenchymal stem cell transplantation can restore lupus disease-associated miRNA expression and Th1/Th2 ratios in a murine model of SLE. Science and Reports, 6, 38237.
Di Nicola, M., et al. (2002). Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood, 99(10), 3838–3843.
Li, Y., et al. (2000). Evidence for migration of donor bone marrow stromal cells into recipient thymus after bone marrow transplantation plus bone grafts: A role of stromal cells in positive selection. Experimental Hematology, 28(8), 950–960.
Zhou, Y., et al. (2019). The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. Journal of Clinical Medicine, 8(7).
Chen, X., Armstrong, M. A., & Li, G. (2006). Mesenchymal stem cells in immunoregulation. Immunology and Cell Biology, 84(5), 413–421.
Wang, L., Zhao, Y., & Shi, S. (2012). Interplay between mesenchymal stem cells and lymphocytes: Implications for immunotherapy and tissue regeneration. Journal of Dental Research, 91(11), 1003–1010.
Duffy, M. M., et al. (2011). Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Research & Therapy, 2(4), 34.
Krampera, M., et al. (2003). Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood, 101(9), 3722–3729.
Barda-Saad, M., et al. (1997). Adhesion of thymocytes to bone marrow stromal cells: Regulation by bFGF and IFN-γ. Stem Cells, 15(3), 229–236.
Azghadi, S. M. R., et al. (2016). Mesenchymal stromal cells support the viability and differentiation of thymocytes through direct contact in autologous co-cultures. Histochemistry and cell biology, 146(2), 153–165.
Small, T., et al. (1999). Comparison of immune reconstitution after unrelated and related T-cell–depleted bone marrow transplantation: Effect of patient age and donor leukocyte infusions. Blood, The Journal of the American Society of Hematology, 93(2), 467–480.
Hakim, F. T., et al. (1997). Constraints on CD4 recovery postchemotherapy in adults: Thymic insufficiency and apoptotic decline of expanded peripheral CD4 cells. Blood, The Journal of the American Society of Hematology, 90(9), 3789–3798.
Ren, G., et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell, 2(2), 141–150.
Majumdar, M. K., et al. (2003). Characterization and functionality of cell surface molecules on human mesenchymal stem cells. Journal of Biomedical Science, 10(2), 228–241.
Le Blanc, K., et al. (2003). Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scandinavian journal of immunology, 57(1), 11–20.
Glennie, S., et al. (2005). Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood, 105(7), 2821–2827.
Uccelli, A., Moretta, L., & Pistoia, V. (2006). Immunoregulatory function of mesenchymal stem cells. European journal of immunology, 36(10), 2566–2573.
Li, X., et al. (2016) .Umbilical cord tissue-derived mesenchymal stem cells induce T lymphocyte apoptosis and cell cycle arrest by expression of indoleamine 2, 3-dioxygenase. Stem cells international, 2016.
Benvenuto, F., et al. (2007). Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem cells, 25(7), 1753–1760.
Yamaza, T., et al. (2008). Pharmacologic stem cell based intervention as a new approach to osteoporosis treatment in rodents. PLoS ONE, 3(7), e2615.
Akiyama, K., et al. (2012). Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell, 10(5), 544–555.
Ji, S., et al. (2012). Mesenchymal stem cell transplantation inhibits abnormal activation of Akt/GSK3β signaling pathway in T cells from systemic lupus erythematosus mice. Cellular Physiology and Biochemistry, 29(5–6), 705–712.
Selmani, Z., et al. (2008). Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+ CD25highFOXP3+ regulatory T cells. Stem cells, 26(1), 212–222.
Quaedackers, M. E., et al. (2009). Cell contact interaction between adipose-derived stromal cells and allo-activated T lymphocytes. European Journal of Immunology, 39(12), 3436–3446.
Aggarwal, S., & Pittenger, M. F. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood, 105(4), 1815–1822.
Shi, Y., et al. (2012). How mesenchymal stem cells interact with tissue immune responses. Trends in immunology, 33(3), 136–143.
Rasmusson, I., et al. (2003). Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation, 76(8), 1208–1213.
Hof-Nahor, I., et al. (2012). Human mesenchymal stem cells shift CD8+ T cells towards a suppressive phenotype by inducing tolerogenic monocytes. Journal of Cell Science, 125(19), 4640–4650.
Weng, N.-P., Araki, Y., & Subedi, K. (2012). The molecular basis of the memory T cell response: Differential gene expression and its epigenetic regulation. Nature Reviews Immunology, 12(4), 306–315.
Luque-Campos, N., et al. (2019). Mesenchymal stem cells improve rheumatoid arthritis progression by controlling memory T cell response. Frontiers in immunology, 10, 798.
Pianta, S., et al. (2015). Amniotic membrane mesenchymal cells-derived factors skew T cell polarization toward Treg and downregulate Th1 and Th17 cells subsets. Stem Cell Reviews and Reports, 11(3), 394–407.
Haddad, R., & Saldanha-Araujo, F. (2014). Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: What do we know so far? BioMed Research International, 2014, 216806.
Augello, A., et al. (2005). Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. European Journal of Immunology, 35(5), 1482–1490.
Yi, T., & Song, S. U. (2012). Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Archives of Pharmacal Research, 35(2), 213–221.
Sharabi, A., et al. (2018). Regulatory T cells in the treatment of disease. Nature Reviews Drug Discovery, 17(11), 823–844.
Chen, W., et al. (2003). Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. The Journal of experimental medicine, 198(12), 1875–1886.
Romano, M., et al. (2019). Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Frontiers in immunology, 10, 43.
Prevosto, C., et al. (2007). Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica, 92(7), 881–888.
Gu, Z., et al. (2010). Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus, 19(13), 1502–1514.
Choi, E. W., et al. (2012). Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue–derived mesenchymal stem cell transplantation. Arthritis & Rheumatism, 64(1), 243–253.
Shi, M., Liu, Z. W., & Wang, F. S. (2011). Immunomodulatory properties and therapeutic application of mesenchymal stem cells. Clinical and experimental immunology, 164(1), 1–8.
Nam, Y., et al. (2018). Intraperitoneal infusion of mesenchymal stem cell attenuates severity of collagen antibody induced arthritis. PLoS ONE, 13(6), e0198740.
Roux, C., et al. (2018). Immunosuppressive mesenchymal stromal cells derived from human-induced pluripotent stem cells induce human regulatory T cells in vitro and in vivo. Frontiers in immunology, 8, 1991.
Hong, J. W., et al. (2017). Immune tolerance of human dental pulp-derived mesenchymal stem cells mediated by CD4+ CD25+ FoxP3+ regulatory T-cells and induced by TGF-β1 and IL-10. Yonsei Medical Journal, 58(5), 1031.
Svobodova, E., et al. (2012). The role of mouse mesenchymal stem cells in differentiation of naive T-cells into anti-inflammatory regulatory T-cell or proinflammatory helper T-cell 17 population. Stem cells and development, 21(6), 901–910.
He, J.-G., et al. (2020). Indoleamine 2, 3-dioxgenase-transfected mesenchymal stem cells suppress heart allograft rejection by increasing the production and activity of dendritic cells and regulatory T cells. Journal of Investigative Medicine, 68(3), 728–737.
Mougiakakos, D., et al. (2011). The impact of inflammatory licensing on heme oxygenase-1–mediated induction of regulatory T cells by human mesenchymal stem cells. Blood, 117(18), 4826–4835.
Chen, C., et al. (2017). Mesenchymal stem cells upregulate Treg cells via sHLA-G in SLE patients. International immunopharmacology, 44, 234–241.
Negi, N., & Griffin, M. D. (2020). Effects of mesenchymal stromal cells on regulatory T cells: Current understanding and clinical relevance. Stem Cells, 38(5), 596–605.
Duffy, M. M., et al. (2011). Mesenchymal stem cell effects on T-cell effector pathways. Stem cell research & therapy, 2(4), 1–9.
Wang, S., Qu, X., & Zhao, R. C. (2012). Clinical applications of mesenchymal stem cells. Journal of hematology & oncology, 5(1), 1–9.
Yang, H.-Y., et al. (2020). Combination product of dermal matrix, human mesenchymal stem cells, and timolol promotes diabetic wound healing in mice. Stem cells translational medicine, 9(11), 1353–1364.
Qiu, X., et al. (2018). Mesenchymal stem cells and extracellular matrix scaffold promote muscle regeneration by synergistically regulating macrophage polarization toward the M2 phenotype. Stem Cell Research & Therapy, 9(1), 1–15.
Del Papa, B., et al. (2013). Notch1 modulates mesenchymal stem cells mediated regulatory T- cell induction. European Journal of Immunology, 43(1), 182–187.
Cahill, E. F., et al. (2015). Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T- cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Research & Therapy, 6(1), 1–13.
Rashedi, I., et al. (2017). TLR3 or TLR4 activation enhances mesenchymal stromal cell-mediated Treg induction via notch signaling. Stem Cells, 35(1), 265–275.
Mota, C., et al. (2014). Delta-like 1–mediated Notch signaling enhances the in vitro conversion of human memory CD4 T cells into FOXP3-expressing regulatory T cells. The Journal of Immunology, 193(12), 5854–5862.
Gu, F., et al. (2014). Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clinical Rheumatology, 33(11), 1611–1619.
Pers, Y.-M., et al. (2018). Injection of adipose-derived stromal cells in the knee of patients with severe osteoarthritis has a systemic effect and promotes an anti-inflammatory phenotype of circulating immune cells. Theranostics, 8(20), 5519.
Martin, J. C., Baeten, D. L., & Josien, R. (2014). Emerging role of IL-17 and Th17 cells in systemic lupus erythematosus. Clinical immunology, 154(1), 1–12.
Xu, S., & Cao, X. (2010). Interleukin-17 and its expanding biological functions. Cellular & molecular immunology, 7(3), 164–174.
Amarilyo, G., et al. (2014). IL-17 promotes murine lupus. The Journal of Immunology, 193(2), 540–543.
Talaat, R. M., et al. (2015). Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematosus (SLE) patients: Correlation with disease activity. Cytokine, 72(2), 146–153.
Wen, Z., et al. (2014). Detection of dynamic frequencies of Th17 cells and their associations with clinical parameters in patients with systemic lupus erythematosus receiving standard therapy. Clinical Rheumatology, 33(10), 1451–1458.
Wong, C., et al. (2000). Elevation of proinflammatory cytokine (IL-18, IL-17, IL-12) and Th2 cytokine (IL-4) concentrations in patients with systemic lupus erythematosus. Lupus, 9(8), 589–593.
Wong, C. K., et al. (2008). Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: Implications for Th17-mediated inflammation in auto-immunity. Clinical immunology, 127(3), 385–393.
Vincent, F. B., et al. (2013). Clinical associations of serum interleukin-17 in systemic lupus erythematosus. Arthritis research & therapy, 15(4), 1–9.
Zhang, Z., Kyttaris, V. C., & Tsokos, G. C. (2009). The role of IL-23/IL-17 axis in lupus nephritis. The Journal of Immunology, 183(5), 3160–3169.
Schmidt, T., et al. (2015). Function of the Th17/interleukin-17A immune response in murine lupus nephritis. Arthritis & rheumatology, 67(2), 475–487.
Pisitkun, P., et al. (2012). Interleukin-17 cytokines are critical in development of fatal lupus glomerulonephritis. Immunity, 37(6), 1104–1115.
Shah, K., et al. (2010). Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis research & therapy, 12(2), 1–10.
Lee, H.-Y., et al. (2008). Altered frequency and migration capacity of CD4+ CD25+ regulatory T cells in systemic lupus erythematosus. Rheumatology, 47(6), 789–794.
Tselios, K., et al. (2014). CD4+ CD25highFOXP3+ T regulatory cells as a biomarker of disease activity in systemic lupus erythematosus: A prospective study. Clinical and Experimental Rheumatology, 32(5), 630–639.
Wang, D., et al. (2017). The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cellular & Molecular Immunology, 14(5), 423–431.
Yang, J., et al. (2011). Recovery of the immune balance between Th17 and regulatory T cells as a treatment for systemic lupus erythematosus. Rheumatology, 50(8), 1366–1372.
Qu, X., et al. (2012). Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Experimental hematology, 40(9), 761–770.
Luz-Crawford, P., et al. (2019). Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem cell research & therapy, 10(1), 1–13.
Ghannam, S., et al. (2010). Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. The Journal of Immunology, 185(1), 302–312.
Shi, M., et al. (2017). A pilot study of mesenchymal stem cell therapy for acute liver allograft rejection. Stem Cells Translational Medicine, 6(12), 2053–2061.
Hsu, H. C., et al. (2008). Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nature Immunology, 9(2), 166–175.
Liu, X., et al. (2015). Mesenchymal stem cells inhibit Th17 cells differentiation via IFN-γ-mediated SOCS3 activation. Immunologic Research, 61(3), 219–229.
Tumangelova-Yuzeir, K., et al. (2019). Mesenchymal stem cells derived and cultured from glioblastoma multiforme increase tregs, downregulate Th17, and induce the tolerogenic phenotype of monocyte-derived cells. Stem Cells Int, 2019, 6904638.
Nurieva, R. I., et al. (2008). Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity, 29(1), 138–149.
Ray, J. P., et al. (2014). Transcription factor STAT3 and type I interferons are corepressive insulators for differentiation of follicular helper and T helper 1 cells. Immunity, 40(3), 367–377.
Zhu, Y., Zou, L., & Liu, Y. C. (2016). T follicular helper cells, T follicular regulatory cells and autoimmunity. International Immunology, 28(4), 173–179.
Fazilleau, N., et al. (2009). Follicular helper T cells: Lineage and location. Immunity, 30(3), 324–335.
McPhee, C. G., et al. (2013). IL-21 is a double-edged sword in the systemic lupus erythematosus-like disease of BXSB.Yaa mice. J Immunol, 191(9), 4581–8.
King, C., Tangye, S. G., & Mackay, C. R. (2008). T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual Review of Immunology, 26, 741–766.
Johnston, R. J., et al. (2009). Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science, 325(5943), 1006–1010.
Choi, J. Y., et al. (2015). Circulating follicular helper-like T cells in systemic lupus erythematosus: Association with disease activity. Arthritis & Rhematology, 67(4), 988–999.
Crotty, S. (2014). T follicular helper cell differentiation, function, and roles in disease. Immunity, 41(4), 529–542.
Liu, R., et al. (2015). Allogeneic mesenchymal stem cells inhibited T follicular helper cell generation in rheumatoid arthritis. Science and Reports, 5, 12777.
Liu, R., et al. (2015). Umbilical cord mesenchymal stem cells inhibit the differentiation of circulating T follicular helper cells in patients with primary Sjögren’s syndrome through the secretion of indoleamine 2,3-dioxygenase. Rheumatology (Oxford), 54(2), 332–342.
Yang, X., et al. (2018). Bone marrow-derived mesenchymal stem cells inhibit T follicular helper cell in lupus-prone mice. Lupus, 27(1), 49–59.
Jang, E., et al. (2016). Infusion of human bone marrow-derived mesenchymal stem cells alleviates autoimmune nephritis in a lupus model by suppressing follicular helper T-cell development. Cell Transplantation, 25(1), 1–15.
Brady, M. T., et al. (2014). Mesenchymal stromal cells support the viability and differentiation of follicular lymphoma-infiltrating follicular helper T-cells. PLoS ONE, 9(5), e97597.
Lee, H. J., et al. (2017). ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells. Science and Reports, 7, 44486.
Chung, Y., et al. (2011). Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature Medicine, 17(8), 983–988.
Linterman, M. A., et al. (2011). Foxp3+ follicular regulatory T cells control the germinal center response. Nature Medicine, 17(8), 975–982.
Yang, X., et al. (2013). T follicular helper cells mediate expansion of regulatory B cells via IL-21 in Lupus-prone MRL/lpr mice. PLoS ONE, 8(4), e62855.
Yang, X., et al. (2014). T follicular helper cells and regulatory B cells dynamics in systemic lupus erythematosus. PLoS ONE, 9(2), e88441.
Spolski, R., et al. (2009). IL-21 mediates suppressive effects via its induction of IL-10. The Journal of Immunology, 182(5), 2859–2867.
Wollenberg, I., et al. (2011). Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. The Journal of Immunology, 187(9), 4553–4560.
Nagy, G., et al. (2000). Measurement of intracellular interferon-gamma and interleukin-4 in whole blood T lymphocytes from patients with systemic lupus erythematosus. Immunology Letters, 74(3), 207–210.
Youd, M., et al. (2010). Allogeneic mesenchymal stem cells do not protect NZBxNZW F1 mice from developing lupus disease. Clinical and Experimental Immunology, 161(1), 176–186.
Keyhanmanesh, R., et al. (2018). Systemic delivery of mesenchymal stem cells condition media in repeated doses acts as magic bullets in restoring IFN-γ/IL-4 balance in asthmatic rats. Life Sciences, 212, 30–36.
Ezquer, F., et al. (2012). The antidiabetic effect of mesenchymal stem cells is unrelated to their transdifferentiation potential but to their capability to restore Th1/Th2 balance and to modify the pancreatic microenvironment. Stem Cells, 30(8), 1664–1674.
Lim, J. Y., et al. (2014). Combination cell therapy using mesenchymal stem cells and regulatory T-cells provides a synergistic immunomodulatory effect associated with reciprocal regulation of TH1/TH2 and th17/treg cells in a murine acute graft-versus-host disease model. Cell Transplantation, 23(6), 703–714.
Akahoshi, M., et al. (1999). Th1/Th2 balance of peripheral T helper cells in systemic lupus erythematosus. Arthritis and Rheumatism, 42(8), 1644–1648.
Sun, L., et al. (2010). Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis and Rheumatism, 62(8), 2467–2475.
Kidd, P. (2003). Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Alternative Medicine Review, 8(3), 223–246.
Gómez, D., et al. (2004). Th1/Th2 cytokines in patients with systemic lupus erythematosus: Is tumor necrosis factor alpha protective? Seminars in Arthritis and Rheumatism, 33(6), 404–413.
Zhang, A., et al. (2020). IL-1β enhances human placenta-derived mesenchymal stromal cells ability to mediate Th1/Th2 and Th1/CD4(+)IL-10(+) T cell balance and regulates its adhesion, proliferation and migration via PD-L1. Cellular Immunology, 352, 104113.
Rad, F., et al. (2019). Mesenchymal stem cell-based therapy for autoimmune diseases: Emerging roles of extracellular vesicles. Molecular Biology Reports, 46(1), 1533–1549.
Bosi, C., Lanzoni, G., & Pugliese, A. (2016). Clinical trials of mesenchymal stem cell transplantation in patients with type 1 diabetes and systemic lupus erythematosus: Is it time for larger studies. CellR4, 4(5), e2134.
Xu, J. (2018). Therapeutic applications of mesenchymal stem cells for systemic lupus erythematosus. Advances in Experimental Medicine and Biology, 1089, 73–85.
Dardalhon, V., et al. (2008). Role of Th1 and Th17 cells in organ-specific autoimmunity. Journal of autoimmunity, 31(3), 252–256.
Batten, P., et al. (2006). Human mesenchymal stem cells induce T cell anergy and downregulate T cell allo-responses via the TH2 pathway: Relevance to tissue engineering human heart valves. Tissue engineering, 12(8), 2263–2273.
Podestà, M. A., Remuzzi, G., & Casiraghi, F. (2019). Mesenchymal stromal cells for transplant tolerance. Frontiers in Immunology, 10, 1287.
Rawat, S., Gupta, S., Mohanty, S. (2019). Mesenchymal stem cells modulate the immune system in developing therapeutic interventions. Immune Response Activation and Immunomodulation.
Najar, M., et al. (2016). Mesenchymal stromal cells and immunomodulation: A gathering of regulatory immune cells. Cytotherapy, 18(2), 160–171.
Gómez, D., et al. (2004). Th1/Th2 cytokines in patients with systemic lupus erythematosus: is tumor necrosis factor α protective? in Seminars in arthritis and rheumatism. Elsevier.
Deng, W., et al. (2005). Effects of allogeneic bone marrow-derived mesenchymal stem cells on T and B lymphocytes from BXSB mice. DNA and cell biology, 24(7), 458–463.
Mauri, C., & Bosma, A. (2012). Immune regulatory function of B cells. Annual review of immunology, 30, 221–241.
Park, M. J., et al. (2015). Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplantation, 24(11), 2367–2377.
Comoli, P., et al. (2008). Human mesenchymal stem cells inhibit antibody production induced in vitro by allostimulation. Nephrology, Dialysis, Transplantation, 23(4), 1196–1202.
Liu, F., et al. (2020). Immunotherapeutic effects of allogeneic mesenchymal stem cells on systemic lupus erythematosus. Lupus, 29(8), 872–883.
Corcione, A., et al. (2006). Human mesenchymal stem cells modulate B-cell functions. Blood, 107(1), 367–372.
Looney, R. J., et al. (2004). B cell depletion as a novel treatment for systemic lupus erythematosus: A phase I/II dose-escalation trial of rituximab. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 50(8), 2580–2589.
Tokoyoda, K., et al. (2004). Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity, 20(6), 707–718.
Asari, S., et al. (2009). Mesenchymal stem cells suppress B-cell terminal differentiation. Experimental hematology, 37(5), 604–615.
Zhang, J., et al. (2001). Cutting edge: A role for B lymphocyte stimulator in systemic lupus erythematosus. The Journal of Immunology, 166(1), 6–10.
Rafei, M., et al. (2008). Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood, 112(13), 4991–4998.
Che, N., et al. (2014). Impaired B cell inhibition by lupus bone marrow mesenchymal stem cells is caused by reduced CCL2 expression. The Journal of Immunology, 193(10), 5306–5314.
Chun, W., Tian, J., & Zhang, Y. (2021). Transplantation of mesenchymal stem cells ameliorates systemic lupus erythematosus and upregulates B10 cells through TGF-β1. Stem Cell Research & Therapy, 12(1), 1–11.
Blair, P. A., et al. (2010). CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity, 32(1), 129–140.
Wu, H.-H., et al. (2019). Mesenchymal stem cell-based drug delivery strategy: From cells to biomimetic. Journal of Controlled Release, 294, 102–113.
Rasmusson, I., et al. (2007). Mesenchymal stem cells stimulate antibody secretion in human B cells. Scandinavian journal of immunology, 65(4), 336–343.
Fillatreau, S., et al. (2002). B cells regulate autoimmunity by provision of IL-10. Nature immunology, 3(10), 944–950.
Carter, N. A., et al. (2011). Mice lacking endogenous IL-10–producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. The Journal of Immunology, 186(10), 5569–5579.
Mauri, C. (2010). CD19 CD24 hi CD38 hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity, 32, 129–140.
Veneri, D., et al. (2008). Peripheral blood CD5-positive B lymphocytes (B-1a cells) after allogeneic stem cell transplantation for acute myeloid leukaemia in humans. Blood Transfusion, 6(4), 220–224.
Peng, Y., et al. (2015). Mesenchymal stromal cells infusions improve refractory chronic graft versus host disease through an increase of CD5+ regulatory B cells producing interleukin 10. Leukemia, 29(3), 636–646.
Soldevila, G., Raman, C., & Lozano, F. (2011). The immunomodulatory properties of the CD5 lymphocyte receptor in health and disease. Current Opinion in Immunology, 23(3), 310–318.
Iwata, Y., et al. (2011). Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood, 117(2), 530–541.
Vadasz, Z., et al. (2013). B-regulatory cells in autoimmunity and immune mediated inflammation. FEBS Letters, 587(13), 2074–2078.
Budoni, M., et al. (2013). The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplantation, 22(2), 369–379.
Rosado, M. M., et al. (2015). Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev, 24(1), 93–103.
Franquesa, M., et al. (2015). Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells independently of T helper cells. Stem Cells, 33(3), 880–891.
Cheema, G. S., et al. (2001). Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis and Rheumatism, 44(6), 1313–1319.
Fan, L., et al. (2016). Interaction between Mesenchymal Stem Cells and B-Cells. International Journal of Molecular Sciences, 17(5).
Wang, Y., et al. (2014). Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nature immunology, 15(11), 1009–1016.
Liu, S., et al. (2019). Treatment of murine lupus with TIGIT-Ig. Clinical immunology, 203, 72–80.
Choi, E. W., et al. (2016). Comparative efficacies of long-term serial transplantation of syngeneic, allogeneic, xenogeneic, or CTLA4Ig-overproducing xenogeneic adipose tissue-derived mesenchymal stem cells on murine systemic Lupus Erythematosus. Cell Transplantation, 25(6), 1193–1206.
Ohl, K., & Tenbrock, K. (2015). Regulatory T cells in systemic Lupus Erythematosus. European journal of immunology, 45(2), 344–355.
Tsokos, G. C., et al. (2016). New insights into the immunopathogenesis of systemic Lupus Erythematosus. Nature Reviews Rheumatology, 12(12), 716–730.
Brunstein, C. G., et al. (2011). Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood, The Journal of the American Society of Hematology, 117(3), 1061–1070.
Marek-Trzonkowska, N., et al. (2013). Clinical application of regulatory T cells in type 1 diabetes. Pediatric diabetes, 14(5), 322–332.
Issa, F., & Wood, K. J. (2010). CD4+ regulatory T cells in solid organ transplantation. Current opinion in organ transplantation, 15(6), 757.
Kasper, I. R., et al. (2016). Empowering regulatory T cells in autoimmunity. Trends in molecular medicine, 22(9), 784–797.
He, J., et al. (2016). Low-dose interleukin-2 treatment selectively modulates CD4+ T cell subsets in patients with systemic Lupus Erythematosus. Nature medicine, 22(9), 991–993.
Koga, T., et al. (2012). Calcium/calmodulin-dependent protein kinase IV suppresses IL-2 production and regulatory T cell activity in lupus. The Journal of Immunology, 189(7), 3490–3496.
Serakinci, N., Fahrioglu, U., & Christensen, R. (2014). Mesenchymal stem cells, cancer challenges and new directions. European Journal of Cancer, 50(8), 1522–1530.
Hagiwara, M., et al. (2008). Kallikrein-modified mesenchymal stem cell implantation provides enhanced protection against acute ischemic kidney injury by inhibiting apoptosis and inflammation. Human gene therapy, 19(8), 807–819.
Mangi, A. A., et al. (2003). Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nature medicine, 9(9), 1195–1201.
Li, Y., et al. (2014). Delivering oxidation resistance-1 (OXR1) to mouse kidney by genetic modified mesenchymal stem cells exhibited enhanced protection against nephrotoxic serum induced renal injury and lupus nephritis. Journal of stem cell research & therapy, 4(9).
Li, Y., et al. (2013). Kallikrein transduced mesenchymal stem cells protect against anti-GBM disease and lupus nephritis by ameliorating inflammation and oxidative stress. PLoS ONE, 8(7), e67790.
Diaz, M. F., et al. (2017). Biomechanical forces promote immune regulatory function of bone marrow mesenchymal stromal cells. Stem Cells, 35(5), 1259–1272.
Zhang, Z., et al. (2019). Clearance of apoptotic cells by mesenchymal stem cells contributes to immunosuppression via PGE2. eBioMedicine, 45, 341–350.
Huang, S., et al. (2016). No significant effects of Poly (I: C) on human umbilical cord-derived mesenchymal stem cells in the treatment of B6. MRL-Faslpr mice. Current Research in Translational Medicine, 64(2), 55–60.
Mastri, M., et al. (2012). Activation of Toll-like receptor 3 amplifies mesenchymal stem cell trophic factors and enhances therapeutic potency. American Journal of Physiology-Cell Physiology, 303(10), C1021–C1033.
Szabó, E., et al. (2015). Licensing by inflammatory cytokines abolishes heterogeneity of immunosuppressive function of mesenchymal stem cell population. Stem Cells and Development, 24(18), 2171–2180.
Boland, L., et al. (2018). IFN-γ and TNF-α pre-licensing protects mesenchymal stromal cells from the pro-inflammatory effects of palmitate. Molecular Therapy, 26(3), 860–873.
Nozari, P., et al. (2022). Investigation of the effect of IFN-γ/TNF-α-treated mesenchymal stem cells on Th9-and Treg cell-related parameters in a mouse model of ovalbumin-induced allergic asthma. Immunopharmacology and Immunotoxicology, (just-accepted): 1–26.
Hackel, A., et al. (2021). TNF-α and IL-1β sensitize human MSC for IFN-γ signaling and enhance neutrophil recruitment. European Journal of Immunology, 51(2), 319–330.
Polchert, D., et al. (2008). IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. European journal of immunology, 38(6), 1745–1755.
Han, X., et al. (2014). Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death & Differentiation, 21(11), 1758–1768.
Waterman, R. S., et al. (2010). A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE, 5(4), e10088.
Elloumi, N., et al. (2021). RNA receptors, TLR3 and TLR7, are potentially associated with SLE clinical features. International Journal of Immunogenetics, 48(3), 250–259.
Petri, M., et al. (2013). Vitamin D in systemic Lupus Erythematosus: Modest association with disease activity and the urine protein-to-creatinine ratio. Arthritis & Rheumatism, 65(7), 1865–1871.
Dall’Ara, F., et al. (2017). Vitamin D and systemic lupus erythematous: A review of immunological and clinical aspects. Clinical and experimental rheumatology, 36(1), 153–162.
Terrier, B., et al. (2012). Restoration of regulatory and effector T cell balance and B cell homeostasis in systemic Lupus Erythematosus patients through vitamin D supplementation. Arthritis research & therapy, 14(5), 1–10.
Shahin, D., et al. (2017). Serum 25-OH vitamin D level in treatment-naïve systemic Lupus Erythematosus patients: Relation to disease activity, IL-23 and IL-17. Lupus, 26(9), 917–926.
Geng, S., et al. (2013). Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells. Metabolism, 62(6), 768–777.
Andrukhov, O., et al. (2020). Vitamin D3 and dental mesenchymal stromal cells. Applied Sciences, 10(13), 4527.
Salehpour, A., et al. (2021). 1, 25-Dihydroxyvitamin D3 modulates adipogenesis of human adipose-derived mesenchymal stem cells dose-dependently. Nutrition & metabolism, 18(1), 1–9.
Xu, J.-J., et al. (2015). Vitamin D analog EB1089 could repair the defective bone marrow-derived mesenchymal stromal cells in patients with systemic Lupus Erythematosus. International Journal of Clinical and Experimental Medicine, 8(1), 916.
Hou, Y.-C., et al. (2020). The role of vitamin D in modulating mesenchymal stem cells and endothelial progenitor cells for vascular calcification. International Journal of Molecular Sciences, 21(7), 2466.
Blufstein, A., et al. (2021). Effect of vitamin D3 on the osteogenic differentiation of human periodontal ligament stromal cells under inflammatory conditions. Journal of Periodontal Research, 56(3), 579–588.
Motlagh, B. M., Froushani, S. M. A., Ahangaran, N. A. (2017). Vitamin D3 modifies the impacts of the supernatants of mesenchymal stem cells on macrophages functions. Zahedan Journal of Research in Medical Sciences, 19(6).
Hamza, R. Z., Al-Eisa, R. A., & El-Shenawy, N. S. (2021). Efficacy of mesenchymal stem cell and vitamin D in the treatment of diabetes mellitus induced in a rat model: Pancreatic tissues. Coatings, 11(3), 317.
McMurray, R. W., & May, W. (2003). Sex hormones and systemic Lupus Erythematosus: Review and meta-analysis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology, 48(8), 2100–2110.
Olsen, N. J., & Kovacs, W. J. (1995). Case report: Testosterone treatment of systemic Lupus Erythematosus in a patient with Klinefelter’s syndrome. The American journal of the medical sciences, 310(4), 158–160.
Yung, R. (1999). Mechanisms of lupus: The role of estrogens. Clinical and experimental rheumatology, 17, 271–275.
Schmohl, K. A., et al. (2020). Thyroid hormone effects on mesenchymal stem cell biology in the tumour microenvironment. Experimental and Clinical Endocrinology & Diabetes, 128(06/07), 462–468.
Munoz, L. E., et al. (2010). The role of defective clearance of apoptotic cells in systemic autoimmunity. Nature Reviews Rheumatology, 6(5), 280–289.
Lee, C. S., et al. (2016). Boosting apoptotic cell clearance by colonic epithelial cells attenuates inflammation in vivo. Immunity, 44(4), 807–820.
Najafian, N., & Sayegh, M. H. (2000). CTLA4-Ig: A novel immunosuppressive agent. Expert opinion on investigational drugs, 9(9), 2147–2157.
Abrams, J. R., et al. (1999). CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. The Journal of clinical investigation, 103(9), 1243–1252.
Ceccarelli, F., et al. (2015). Assessment of disease activity in systemic Lupus Erythematosus: Lights and shadows. Autoimmunity reviews, 14(7), 601–608.
Mattar, P., & Bieback, K. (2015). Comparing the immunomodulatory properties of bone marrow, adipose tissue, and birth-associated tissue mesenchymal stromal cells. Frontiers in immunology, 6, 560.
Youd, M., et al. (2010). Allogeneic mesenchymal stem cells do not protect NZB× NZW F1 mice from developing lupus disease. Clinical & Experimental Immunology, 161(1), 176–186.
Shahror, R.A., et al. (2020) Genetically modified mesenchymal stem cells: the next generation of stem cell-based therapy for TBI. International Journal of Molecular Sciences, 21 (11)
Rostami, M., Haidari, K., & Shahbazi, M. (2018). Genetically engineered adipose mesenchymal stem cells using hiv-based lentiviral vectors as gene therapy for autoimmune diseases Cell Reprogram, 20(6), 337–346.
Author information
Authors and Affiliations
Contributions
Akram Hoseinzadeh has drafted the work.
Mahmoud Mahmoudi, Zahra Rezaieyazdi, Jalil Tavakol Afshari, Seyed-Alireza Esmaeili and Reza Moradi thoroughly reviewed it.
Ali Mahmoudi and Sahar Heydari have drawn the figures.
All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
“Not applicable”.
Consent for Publication
“Not applicable”.
Competing Interests
All authors declare that they have no competing of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hoseinzadeh, A., Rezaieyazdi, Z., Afshari, J.T. et al. Modulation of Mesenchymal Stem Cells-Mediated Adaptive Immune Effectors’ Repertoire in the Recovery of Systemic Lupus Erythematosus. Stem Cell Rev and Rep 19, 322–344 (2023). https://doi.org/10.1007/s12015-022-10452-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10452-7