Abstract
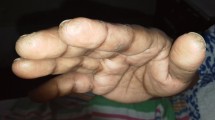
Anti-Jo-1 is the most frequently detectable antibody in the antisynthetase syndrome (ASSD), an autoimmune disease characterized by the occurrence of arthritis, myositis, and interstitial lung disease (ILD). Recently, we organized an international collaborative group called American and European NEtwork of Antisynthetase Syndrome (AENEAS) for the study of this rare and fascinating disease. The group collected and published one of the largest series of ASSD patients ever described and with one of the longer follow-up ever reported. The number of participating centers is steadily increasing, as well as the available cohort. In the first paper, we showed that arthritis, myositis, and ILD may be frequently the only feature at disease onset, raising problems to reach a correct diagnosis of this syndrome. Nevertheless, we first observed that the ex novo appearance of further manifestations is common during the follow-up, strengthening the importance of a correct diagnosis. In our cohort, the 24 % of the 243 patients up to now collected had isolated arthritis as a presenting feature. These patients represent the most intriguing group in terms of differential diagnosis and clinical time course. Furthermore, data on this aspect are scanty, the reason that lead us to evaluate these aspects in our cohort of patients, reviewing also available literature. In fact, the most relevant aspect is that ASSD is rarely suspected in this setting of patients, in particular in case of poliarticular involvement, positive rheumatoid factor (RF), or anti-cyclic citrullinated peptide antibodies (ACPA) or evidence of joint erosions at plain radiographs. These findings were not rare in our cohort, and they have been also described in other series. Furthermore, manifestations such as Raynaud’s phenomenon, mechanic’s hands, and fever that may lead to the suspect of ASSD are observed only in a third of cases. If we consider the high rate of clinical picture progression in these patients, we feel that ASSD should be carefully considered in all patients presenting with isolated arthritis, even in those with erosive, RF, and ACPA-positive arthritis.

Similar content being viewed by others
References
Imbert-Masseau A, Hamidou M, Agard C, Grolleau JY, Cherin P (2003) Antisynthetase syndrome. Joint Bone Spine 70:161–168
Milisenda JC, Selva-O’Callaghan A, Grau JM (2014) The diagnosis and classification of polymyositis. J Autoimmun 48-49:118–121
Jeganathan V, Peeva E, Diamond B (2014) Hormonal milieu at time of B cell activation controls duration of autoantibody response. J Autoimmun 53:46–54
Tang X, Zhang B, Jarrell JA, Price JV, Dai H, Utz PJ et al (2014) Ly108 expression distinguishes subsets of invariant NKT cells that help autoantibody production and secrete IL-21 from those that secrete IL-17 in lupus prone NZB/W mice. J Autoimmun 50:87–98
Betteridge Z, McHugh N (2015) Myositis-specific autoantibodies: an important tool to support diagnosis of myositis. J Intern Med (in press)
Garcia-De La Torre I (2015) Clinical usefulness of autoantibodies in idiopathic inflammatory myositis. Front Immunol 6:331
Selmi C, Ceribelli A, Generali E, Scire CA, Alborghetti F, Colloredo G et al (2015) Serum antinuclear and extractable nuclear antigen antibody prevalence and associated morbidity and mortality in the general population over 15years. Autoimmun Rev 15:162–166
Dugar M, Cox S, Limaye V, Blumbergs P, Roberts-Thomson PJ (2011) Clinical heterogeneity and prognostic features of South Australian patients with anti-synthetase autoantibodies. Intern Med J 41:674–679
Hervier B, Devilliers H, Stanciu R, Meyer A, Uzunhan Y, Masseau A et al (2012) Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev 12:210–217
Chatterjee S, Prayson R, Farver C (2013) Antisynthetase syndrome: not just an inflammatory myopathy. Cleve Clin J Med 80:655–666
Cavagna L, Nuno L, Scire CA, Govoni M, Longo FJ, Franceschini F et al (2015) Clinical spectrum time course in anti Jo-1 positive antisynthetase syndrome: results from an international retrospective multicenter study. Medicine (Baltimore) 94:e1144
Hervier B, Benveniste O (2013) Clinical heterogeneity and outcomes of antisynthetase syndrome. Curr Rheumatol Rep 15:349
Cavagna L, Caporali R, Abdi-Ali L, Dore R, Meloni F, Montecucco C (2013) Cyclosporine in anti-Jo1-positive patients with corticosteroid-refractory interstitial lung disease. J Rheumatol 40:484–492
Ghirardello A, Zampieri S, Tarricone E, Iaccarino L, Bendo R, Briani C et al (2006) Clinical implications of autoantibody screening in patients with autoimmune myositis. Autoimmunity 39:217–221
Nagashima T, Iwamoto M, Minota S (2011) Antisynthetase syndrome associated with long-standing rheumatoid arthritis. Rheumatol Int 31:705–706
Cavagna L, Fusetti C, Montecucco C, Caporali R (2010) Anticyclic citrullinated peptide antibodies as markers of erosive arthritis in antisynthetase syndrome. J Rheumatol 37:1967, author reply 8
Kaneko Y, Hanaoka H, Hirakata M, Takeuchi T, Kuwana M (2014) Distinct arthropathies of the hands in patients with anti-aminoacyl tRNA synthetase antibodies: usefulness of autoantibody profiles in classifying patients. Rheumatology (Oxford) 53:1120–1124
Chan MT, Owen P, Dunphy J, Cox B, Carmichael C, Korendowych E et al (2008) Associations of erosive arthritis with anti-cyclic citrullinated peptide antibodies and MHC Class II alleles in systemic lupus erythematosus. J Rheumatol 35:77–83
Gomard-Mennesson E, Fabien N, Cordier JF, Ninet J, Tebib J, Rousset H (2007) Clinical significance of anti-histidyl-tRNA synthetase (Jo1) autoantibodies. Ann N Y Acad Sci 1109:414–420
Park CK, Kim TJ, Cho YN, Kim IS, Lee HJ, Lee KE et al (2011) Development of antisynthetase syndrome in a patient with rheumatoid arthritis. Rheumatol Int 31:529–532
Bunch TW, O’Duffy JD, McLeod RA (1976) Deforming arthritis of the hands in polymyositis. Arthritis Rheum 19:243–248
Greenway G, Weisman MH, Resnick D, Zvaifler NJ, Guerra J Jr (1981) Deforming arthritis of the hands: an unusual manifestation of polymyositis. AJR Am J Roentgenol 136:611–612
Labrador-Horrillo M, Martinez MA, Selva-O’Callaghan A, Delgado JF, Martinez-Gomez X, Trallero-Araguas E et al (2009) Anti-cyclic citrullinated peptide and anti-keratin antibodies in patients with idiopathic inflammatory myopathy. Rheumatology (Oxford) 48:676–679
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Nagashima T, Sato H, Minota S (2009) Destructive arthropathy associated with dermatomyositis sine myositis positive for anti-Jo-1 and anti-cyclic citrullinated peptide antibodies. J Rheumatol 36:2133–2134
Schmidt WA, Wetzel W, Friedlander R, Lange R, Sorensen HF, Lichey HJ et al (2000) Clinical and serological aspects of patients with anti-Jo-1 antibodies—an evolving spectrum of disease manifestations. Clin Rheumatol 19:371–377
Meyer O, Charlanne H, Cherin P, Allanore Y, Coquerelle P, Grardel B et al (2009) Subluxing arthropathy: an unusual manifestation of the antisynthetase syndrome. Ann Rheum Dis 68:152–153
Nagashima T, Onishi S, Kamata Y, Minota S (2009) Dermatomyositis associated with thyroid cancer: a paraneoplastic syndrome? Rheumatol Int 29:1261–1262
Lefevre G, Meyer A, Launay D, Machelart I, DeBandt M, Michaud J et al (2015) Seronegative polyarthritis revealing antisynthetase syndrome: a multicentre study of 40 patients. Rheumatology (Oxford) 54:927–932
Meyer A, Lefevre G, Bierry G, Duval A, Ottaviani S, Meyer O et al (2015) In antisynthetase syndrome, ACPA are associated with severe and erosive arthritis: an overlapping rheumatoid arthritis and antisynthetase syndrome. Medicine (Baltimore) 94:e523
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd et al (2010) 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 69:1580–1588
Mumm GE, McKown KM, Bell CL (2010) Antisynthetase syndrome presenting as rheumatoid-like polyarthritis. J Clin Rheumatol 16:307–312
Aggarwal R, Cassidy E, Fertig N, Koontz DC, Lucas M, Ascherman DP et al (2014) Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 73:227–232
D’Amico F, Skarmoutsou E, Mazzarino MC (2014) The sex bias in systemic sclerosis: on the possible mechanisms underlying the female disease preponderance. Clin Rev Allergy Immunol 47:334–343
Moutsopoulos HM (2014) Sjogren’s syndrome: a forty-year scientific journey. J Autoimmun 51:1–9
Yu C, Gershwin ME, Chang C (2014) Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun 48-49:10–13
Valim V, Trevisani VF, de Sousa JM, Vilela VS, Belfort R, Jr (2014) Current approach to dry eye disease. Clin Rev Allergy Immunol 49:288–297
Borchers AT, Gershwin ME (2015) Fibromyalgia: a critical and comprehensive review. Clin Rev Allergy Immunol 49:100–151
Gunawardena H (2015) The clinical features of myositis-associated autoantibodies: a review. Clin Rev Allergy Immunol (in press)
Satoh M, Tanaka S, Ceribelli A, Calise SJ, Chan EK (2015) A comprehensive overview on myositis-specific antibodies: new and old biomarkers in idiopathic inflammatory myopathy. Clin Rev Allergy Immunol (in press)
Connors GR, Christopher-Stine L, Oddis CV, Danoff SK (2010) Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 138:1464–1474
Cavagna L, Gonzalez-Gay MA, Castaneda-Sanz S, Franceschini F, Airo P, Cavazzana I (2014) Clinical and temporal characterization of anti-Jo-1 positive antisynthetase syndrome: preliminary results of an international multicentre study. Arthritis Rheum 66:550
Song JW, Chung KC (2010) Observational studies: cohort and case-control studies. Plast Reconstr Surg 126:2234–2242
Mann CJ (2003) Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J 20:54–60
Stone KB, Oddis CV, Fertig N, Katsumata Y, Lucas M, Vogt M et al (2007) Anti-Jo-1 antibody levels correlate with disease activity in idiopathic inflammatory myopathy. Arthritis Rheum 56:3125–3131
Cavagna L, Monti S, Grosso V, Boffini N, Scorletti E, Crepaldi G et al (2013) The multifaceted aspects of interstitial lung disease in rheumatoid arthritis. Biomed Res Int 2013:759760
Ishikawa Y, Yukawa N, Ohmura K, Hosono Y, Imura Y, Kawabata D et al (2010) Etanercept-induced anti-Jo-1-antibody-positive polymyositis in a patient with rheumatoid arthritis: a case report and review of the literature. Clin Rheumatol 29:563–566
Musial J, Undas A, Celinska-Lowenhoff M (2003) Polymyositis associated with infliximab treatment for rheumatoid arthritis. Rheumatology (Oxford) 42:1566–1568
Urata Y, Wakai Y, Kowatari K, Nitobe T, Mizushima Y (2006) Polymyositis associated with infliximab treatment for rheumatoid arthritis. Mod Rheumatol 16:410–411
Tansley S, Gunawardena H (2014) The evolving spectrum of polymyositis and dermatomyositis—moving towards clinicoserological syndromes: a critical review. Clin Rev Allergy Immunol 47:264–273
Iaccarino L, Ghirardello A, Bettio S, Zen M, Gatto M, Punzi L et al (2014) The clinical features, diagnosis and classification of dermatomyositis. J Autoimmun 48-49:122–127
Marie I, Josse S, Decaux O, Dominique S, Diot E, Landron C et al (2012) Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev 11:739–745
Johnson C, Connors GR, Oaks J, Han S, Truong A, Richardson B et al (2014) Clinical and pathologic differences in interstitial lung disease based on antisynthetase antibody type. Respir Med 108:1542–1548
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Cavagna, L., Nuño, L., Scirè, C.A. et al. Serum Jo-1 Autoantibody and Isolated Arthritis in the Antisynthetase Syndrome: Review of the Literature and Report of the Experience of AENEAS Collaborative Group. Clinic Rev Allerg Immunol 52, 71–80 (2017). https://doi.org/10.1007/s12016-016-8528-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-016-8528-9