Abstract
Background
Fibroblast growth factor 21 (FGF21) is expressed in several metabolically active tissues, including liver, fat, and acinar pancreas, and has pleiotropic effects on metabolic homeostasis. The dominant source of FGF21 in the circulation is the liver.
Objective and methods
To analyze the physiological functions of hepatic FGF21, we generated a hepatocyte-specific knockout model (LKO) by mating albumin-Cre mice with FGF21 flox/flox (fl/fl) mice and challenged it with different nutritional models.
Results
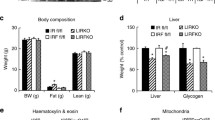
Mice fed a ketogenic diet typically show increased energy expenditure; this effect was attenuated in LKO mice. LKO on KD also developed hepatic pathology and altered hepatic lipid homeostasis. When evaluated using hyperinsulinemic-euglycemic clamps, glucose infusion rates, hepatic glucose production, and glucose uptake were similar between fl/fl and LKO DIO mice.
Conclusions
We conclude that liver-derived FGF21 is important for complete adaptation to ketosis but has a more limited role in the regulation of glycemic homeostasis.





Similar content being viewed by others
Abbreviations
- FGF21:
-
Fibroblast growth factor 21
- fl/fl:
-
Flox/flox
- LKO:
-
FGF21 liver-specific knockout
- KD:
-
Ketogenic diet
- HFD:
-
High fat diet
- PPAR α:
-
Peroxisome proliferator-activated receptor α
- FGF21KO:
-
Fibroblast growth factor 21 knockout
- WT:
-
Wild type
- IP:
-
Intraperitoneal
- [2-14C]D-G:
-
[2-14C]D-glucose
- RG :
-
Glucose metabolic Index
- SEM:
-
Standard error of the mean
- CLAMS:
-
Comprehensive lab animal monitoring system oxymax
- qNMR:
-
Quantitative nuclear magnetic resonance
- ALT:
-
Alanine aminotransferase
- PGK:
-
PhosphoGlycerate Kinase
- NAFLD:
-
Nonalcoholic fatty liver disease
- BAT:
-
Brown adipose tissue
- UCP1:
-
Uncoupling protein 1
- SNA:
-
Sympathetic nerve activity
- ANOVA:
-
Analysis of variance
- KLB:
-
β-Klotho
- EWAT:
-
Epididymal white adipose tissue
- IWAT:
-
Inguinal white adipose tissue
- ipGTT:
-
Intraperitoneal glucose tolerance test
- ITT:
-
Insulin tolerance test
- RER:
-
Respiratory exchange ratio
- MCD:
-
Methionine choline deficient
- SNS:
-
Sympathetic nervous system
- DIO:
-
Diet induced obesity
- FAS:
-
Fatty acids synthase
- SCD-1:
-
Stearoyl-CoA desaturase
- CD36:
-
Cluster of differentiation 36
- LCAD:
-
Long chain acyl-CoA dehydrogenase
- VLCAD:
-
Very long chain acyl-CoA dehydrogenase
- ACOX1:
-
Peroxisomal acyl-coenzyme A oxidase 1
- CPT1α:
-
Carnitine palmitoyltransferase I α
- PPAR:
-
Peroxisome proliferator-activated receptor
- PGC1 α:
-
Peroxisome proliferator-activated receptor (PPAR) γ Coactivator 1α
- PGC1 β:
-
Peroxisome proliferator-activated receptor (PPAR) γ Coactivator 1β
- ABCG5:
-
ATP-binding cassette sub-family G member 5
- ABCG8:
-
ATP-binding cassette sub-family G member 8
- LXR:
-
Liver X receptor
- Cyp7A1:
-
Cytochrome P450 7A1,
- SHP:
-
Small heterodimer partner
- HMGCS1:
-
3-hydroxy-3-methylglutaryl-CoA synthase
- HMGCR:
-
3-hydroxy-3-methylglutaryl-CoA reductase
- SREBP2:
-
Sterol regulatory element-binding protein 2
- PCSK9:
-
Proprotein convertase subtilisin/kexin type 9
- LDLR:
-
LDL receptor
- TGF1 β:
-
Transforming growth factor 1 β
- MCP1:
-
Monocyte chemoattractant protein-1
- MMP2:
-
Matrix metalloproteinase-2
- SMA:
-
Smooth muscle actin
- IL1 β:
-
Interleukin 1 β
- TIMP:
-
Metallopeptidase inhibitor 1
References
T. Inagaki, M. Choi, A. Moschetta, L. Peng, C.L. Cummins, J.G. McDonald et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2, 217–225 (2005)
T. Shimada, S. Mizutani, T. Muto, T. Yoneya, R. Hino, S. Takeda et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl Acad. Sci. USA 98, 6500–6505 (2001)
T. Shimada, Y. Yamazaki, M. Takahashi, H. Hasegawa, I. Urakawa, T. Oshima et al. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Ren. Physiol. 289, F1088–F1095 (2005)
A. Kharitonenkov, T.L. Shiyanova, A. Koester, A.M. Ford, R. Micanovic, E.J. Galbreath et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 115, 1627–1635 (2005)
T. Coskun, H.A. Bina, M.A. Schneider, J.D. Dunbar, C.C. Hu, Y. Chen et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149, 6018–6027 (2008)
G. Gaich, J.Y. Chien, H. Fu, L.C. Glass, M.A. Deeg, W.L. Holland et al. The effects of LY2405319, an FGF21 analog, in obese human subjects with type 2 diabetes. Cell Metab. 18, 333–340 (2013)
S. Talukdar, Y. Zhou, D. Li, M. Rossulek, J. Dong, V. Somayaji et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab. 23, 427–440 (2016)
J. Xu, S. Stanislaus, N. Chinookoswong, Y.Y. Lau, T. Hager, J. Patel et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models-association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab. 297, E1105–E1114 (2009)
M.K. Badman, P. Pissios, A.R. Kennedy, G. Koukos, J.S. Flier, E. Maratos-Flier, Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 5, 426–437 (2007)
T. Inagaki, P. Dutchak, G. Zhao, X. Ding, L. Gautron, V. Parameswara et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 5, 415–425 (2007)
K.J.P. Schwenger, S.E. Fischer, T.D. Jackson, A. Okrainec, J.P. Allard, Non-alcoholic fatty liver disease in morbidly obese individuals undergoing bariatric surgery: prevalence and effect of the pre-bariatric very low calorie diet. Obes. Surg. 28, 1109–1116 (2018)
F.M. Fisher, P.C. Chui, I.A. Nasser, Y. Popov, J.C. Cunniff, T. Lundasen et al. Fibroblast growth factor 21 limits lipotoxicity by promoting hepatic fatty acid activation in mice on methionine and choline-deficient diets. Gastroenterology 147(1073–1083), e1076 (2014)
M.J. Potthoff, T. Inagaki, S. Satapati, X. Ding, T. He, R. Goetz et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc. Natl Acad. Sci. USA 106, 10853–10858 (2009)
B.N. Desai, G. Singhal, M. Watanabe, D. Stevanovic, T. Lundasen, F.M. Fisher et al. Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury. Mol. Metab. 6, 1395–1406 (2017)
J.R. Dushay, E. Toschi, E.K. Mitten, F.M. Fisher, M.A. Herman, E. Maratos-Flier, Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab. 4, 51–57 (2015)
F.M. Fisher, M. Kim, L. Doridot, J.C. Cunniff, T.S. Parker, D.M. Levine et al. A critical role for ChREBP-mediated FGF21 secretion in hepatic fructose metabolism. Mol. Metab. 6, 14–21 (2017)
M.K. Badman, A. Koester, J.S. Flier, A. Kharitonenkov, E. Maratos-Flier, Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150, 4931–4940 (2009)
G. Singhal, G. Kumar, S. Chan, F.M. Fisher, Y. Ma, H.G. Vardeh et al. Deficiency of fibroblast growth factor 21 (FGF21) promotes hepatocellular carcinoma (HCC) in mice on a long term obesogenic diet. Mol Metab. 13, 56–66 (2018)
S. Huang, J. He, X. Zhang, Y. Bian, L. Yang, G. Xie et al. Activation of the hedgehog pathway in human hepatocellular carcinomas. Carcinogenesis 27, 1334–1340 (2006)
D. Ye, Y. Wang, H. Li, W. Jia, K. Man, C.M. Lo et al. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1alpha-mediated antioxidant capacity in mice. Hepatology 60, 977–989 (2014)
G. Singhal, F.M. Fisher, M.J. Chee, T.G. Tan, A. El Ouaamari, A.C. Adams et al. Fibroblast growth factor 21 (FGF21) protects against high fat diet induced inflammation and islet hyperplasia in pancreas. PLoS ONE 11, e0148252 (2016)
M.K. Badman, A.R. Kennedy, A.C. Adams, P. Pissios, E. Maratos-Flier, A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independently of weight loss. Am. J. Physiol. Endocrinol. Metab. 297, E1197–E1204 (2009)
N. Douris, T. Melman, J.M. Pecherer, P. Pissios, J.S. Flier, L.C. Cantley et al. Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet. Biochim Biophys. Acta 1852, 2056–2065 (2015)
A.R. Kennedy, P. Pissios, H. Otu, R. Roberson, B. Xue, K. Asakura et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 292, E1724–E1739 (2007)
E.D. Berglund, C.Y. Li, H.A. Bina, S.E. Lynes, M.D. Michael, A.B. Shanafelt et al. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150, 4084–4093 (2009)
J.P. Camporez, F.R. Jornayvaz, M.C. Petersen, D. Pesta, B.A. Guigni, J. Serr et al. Cellular mechanisms by which FGF21 improves insulin sensitivity in male mice. Endocrinology 154, 3099–3109 (2013)
F.M. Fisher, P.C. Chui, P.J. Antonellis, H.A. Bina, A. Kharitonenkov, J.S. Flier et al. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes 59, 2781–2789 (2010)
H. Li, G. Wu, Q. Fang, M. Zhang, X. Hui, B. Sheng et al. Fibroblast growth factor 21 increases insulin sensitivity through specific expansion of subcutaneous fat. Nat. Commun. 9, 272 (2018)
https://www.mousephenotype.org/data/alleles/MGI:1861377/tm1a(EUCOMM)Hmgu. Accessed 19 Jun 2019
J.E. Ayala, D.P. Bracy, C. Malabanan, F.D. James, T. Ansari, P.T. Fueger et al. Hyperinsulinemic-euglycemic clamps in conscious, unrestrained mice. J Vis Exp. (2011)
J.E. Ayala, D.P. Bracy, O.P. McGuinness, D.H. Wasserman, Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55, 390–397 (2006)
K.X. Mulligan, R.T. Morris, Y.F. Otero, D.H. Wasserman, O.P. McGuinness, Disassociation of muscle insulin signaling and insulin-stimulated glucose uptake during endotoxemia. PLoS ONE 7, e30160 (2012)
E.W. Kraegen, D.E. James, A.B. Jenkins, D.J. Chisholm, Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am. J. Physiol. 248, E353–E362 (1985)
G. Singhal, N. Douris, A.J. Fish, X. Zhang, A.C. Adams, J.S. Flier et al. Fibroblast growth factor 21 has no direct role in regulating fertility in female mice. Mol. Metab. 5, 690–698 (2016)
E.M. Brunt, D.E. Kleiner, L.A. Wilson, P. Belt, B.A. Neuschwander-Tetri, N.C.R. Network, Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 53, 810–820 (2011)
S.M. Martinez, G. Crespo, M. Navasa, X. Forns, Noninvasive assessment of liver fibrosis. Hepatology 53, 325–335 (2011)
J. Folch, M. Lees, G.H. Sloane Stanley, A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957)
P. Seoane-Collazo, J. Roa, E. Rial-Pensado, L. Linares-Pose, D. Beiroa, F. Ruiz-Pino et al. SF1-specific AMPKalpha1 deletion protects against diet-induced obesity. Diabetes 67, 2213–2226 (2018)
A. Guilherme, D.J. Pedersen, F. Henriques, A.H. Bedard, E. Henchey, M. Kelly et al. Neuronal modulation of brown adipose activity through perturbation of white adipocyte lipogenesis. Mol. Metab. 16, 116–125 (2018)
K. Shinohara, P. Nakagawa, J. Gomez, D.A. Morgan, N.K. Littlejohn, M.D. Folchert et al. Selective deletion of renin-b in the brain alters drinking and metabolism. Hypertension 70, 990–997 (2017)
A.I. Mina, R.A. LeClair, K.B. LeClair, D.E. Cohen, L. Lantier, A.S. Banks, CalR: a web-based analysis tool for indirect calorimetry experiments. Cell Metab. 28(656–666), e651 (2018)
F.M. Fisher, E. Maratos-Flier, Understanding the physiology of FGF21. Annu Rev. Physiol. 78, 223–241 (2016)
A. Kharitonenkov, R. DiMarchi, FGF21 revolutions: recent advances illuminating FGF21 biology and medicinal properties. Trends Endocrinol. Metab. 26, 608–617 (2015)
N. Douris, D.M. Stevanovic, F.M. Fisher, T.I. Cisu, M.J. Chee, N.L. Nguyen et al. Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology 156, 2470–2481 (2015)
F.M. Fisher, S. Kleiner, N. Douris, E.C. Fox, R.J. Mepani, F. Verdeguer et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012)
N.Y. Kalaany, D.J. Mangelsdorf, LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev. Physiol. 68, 159–191 (2006)
W. Luu, L.J. Sharpe, I.C. Gelissen, A.J. Brown, The role of signalling in cellular cholesterol homeostasis. IUBMB Life 65, 675–684 (2013)
J. Ye, R.A. DeBose-Boyd, Regulation of cholesterol and fatty acid synthesis. Cold Spring Harb. Perspect. Biol 3, 1–14 (2011).
M.M. Chen, C. Hale, S. Stanislaus, J. Xu, M.M. Veniant, FGF21 acts as a negative regulator of bile acid synthesis. J. Endocrinol. 237, 139–152 (2018)
J. Zhang, J. Gupte, Y. Gong, J. Weiszmann, Y. Zhang, K.J. Lee et al. Chronic over-expression of fibroblast growth factor 21 increases bile acid biosynthesis by opposing FGF15/19 action. EBioMedicine 15, 173–183 (2017)
I. Tabas, Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. J. Clin. Invest. 110, 905–911 (2002)
K.R. Markan, M.C. Naber, M.K. Ameka, M.D. Anderegg, D.J. Mangelsdorf, S.A. Kliewer et al. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 63, 4057–4063 (2014)
S. Vernia, J. Cavanagh-Kyros, T. Barrett, C. Tournier, R.J. Davis, Fibroblast growth factor 21 mediates glycemic regulation by hepatic JNK. Cell Rep. 14, 2273–2280 (2016)
J.S. Flier, Irreproducibility of published bioscience research: diagnosis, pathogenesis and therapy. Mol. Metab. 6, 2–9 (2017)
D.J. Drucker, Never waste a good crisis: confronting reproducibility in translational research. Cell Metab. 24, 348–360 (2016)
Acknowledgements
We thank Dr Pavlos Pissios for scientific discussions and Dr Thomas Webb for technical assistance.
Author contributions
G.S., F.M.F., T.C.B., O.P.M., J.S.F. and E.M.F. conceived and designed the experiments. M.W., G.S., J.B., M.M., T.C.B., D.A.M. and R.R. and Vanderbilt MMPC performed the experiments. M.W., G.S., F.M.F., T.C.B., F.S., O.P.M. analyzed the data. E.M.F., J.S.F., F.M.F., K.R. and O.P.M. contributed with reagents/materials/analysis tools/critical revisions. M.W., G.S. and E.M.F. drafted the manuscript.
Funding
This work was supported by NIH Grant DK028082 (to E.M.F. and J.S.F.). M.W. was supported by funds from The Rotary Club of Rome. We are grateful for the help from the HDDC Core supported by NIH Grant NIDDK P30 DK034854. Generation of genetically altered floxed mice was made under the auspices of BADERC 5P30DK057521 and the BNORC 5P30DK046200. K.R was supported by funds from the NIH (HL084207), the American Heart Association (14EIA18860041) and the University of Iowa Fraternal Order of Eagles Diabetes Research Center. O.P.M. was supported by funds from the NIH (DK059637). The Vanderbilt Mouse Metabolic Phenotyping Center (DK059637) and the Hormone Assay and Analytical Services Core (DK059637 and DK020593) provided surgical and analytical support for clamp studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Watanabe, M., Singhal, G., Fisher, F.M. et al. Liver-derived FGF21 is essential for full adaptation to ketogenic diet but does not regulate glucose homeostasis. Endocrine 67, 95–108 (2020). https://doi.org/10.1007/s12020-019-02124-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-019-02124-3