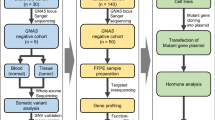
Abstract
Somatic GNAS and USP8 mutations have been implicated in sporadic somatotrophinomas and corticotrophinomas, respectively. However, no genes are known to be recurrently mutated in sporadic prolactinomas. The prevalence of copy number variants (CNV), which is emerging as a mechanism of tumorigenesis in sporadic pituitary adenomas in general, is also unclear in prolactinomas. To characterize the genetic events underpinning sporadic prolactinomas, we performed whole exome sequencing of paired tumor and germline DNA from 12 prolactinoma patients. We observed recurrent large-scale CNV, most commonly in the form of copy number gains. We also identified sequence variants of interest in 15 genes. This included the DRD2, PRL, TMEM67, and MLH3 genes with plausible links to prolactinoma formation. Of the 15 genes of interest, CNV was seen at the gene locus in the corresponding tumor in 10 cases, and pituitary expression of eight genes was in the top 10% of tissues. However, none of our shortlisted somatic variants appeared to be classical driver mutations as no variant was found in more than one tumor. Future directions of research include mechanistic studies to investigate how CNV may contribute to prolactinoma formation, larger studies of relevant prolactinoma subsets according to clinical characteristics, and additional genetic investigations for aberrations not captured by whole exome sequencing.



Similar content being viewed by others
References
Tordjman K, Stern N, Ouaknine G, Yossiphov Y, Razon N, Nordenskjold M, Friedman E (1993) Activating mutations of the Gs alpha-gene in nonfunctioning pituitary tumors. J Clin Endocrinol Metab 77 (3):765–769. https://doi.org/10.1210/jcem.77.3.8396579
Bi WL, Greenwald NF, Ramkissoon SH, Abedalthagafi M, Coy SM, Ligon KL, Mei Y, MacConaill L, Ducar M, Min L, Santagata S, Kaiser UB, Beroukhim R, Laws ER, Jr., Dunn IF (2017) Clinical Identification of Oncogenic Drivers and Copy-Number Alterations in Pituitary Tumors. Endocrinology 158 (7):2284–2291. https://doi.org/10.1210/en.2016-1967
Song ZJ, Reitman ZJ, Ma ZY, Chen JH, Zhang QL, Shou XF, Huang CX, Wang YF, Li SQ, Mao Y, Zhou LF, Lian BF, Yan H, Shi YY, Zhao Y (2016) The genome-wide mutational landscape of pituitary adenomas. Cell Res 26 (11):1255–1259. https://doi.org/10.1038/cr.2016.114
Reincke M, Sbiera S, Hayakawa A, Theodoropoulou M, Osswald A, Beuschlein F (2015) Mutations in the deubiquitinase gene USP8 cause Cushing's disease. Nat Genet 47 (1):31–38. https://doi.org/10.1038/ng.3166
Brastianos PK, Taylor-Weiner A, Manley PE, Jones RT, Dias-Santagata D, Thorner AR, Lawrence MS, Rodriguez FJ, Bernardo LA, Schubert L, Sunkavalli A, Shillingford N, Calicchio ML, Lidov HG, Taha H, Martinez-Lage M, Santi M, Storm PB, Lee JY, Palmer JN, Adappa ND, Scott RM, Dunn IF, Laws ER, Jr., Stewart C, Ligon KL, Hoang MP, Van Hummelen P, Hahn WC, Louis DN, Resnick AC, Kieran MW, Getz G, Santagata S (2014) Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet 46 (2):161–165. https://doi.org/10.1038/ng.2868
Bi WL, Larsen AG, Dunn IF (2018) Genomic Alterations in Sporadic Pituitary Tumors. Curr Neurol Neurosci Rep 18 (1):4. https://doi.org/10.1007/s11910-018-0811-0
Cohen M, Persky R, Stegemann R, Hernandez-Ramirez LC, Zeltser D, Lodish MB, Chen A, Keil MF, Tatsi C, Faucz F, Buchner D, Stratakis CA, Tiosano D (2019) Germline USP8 mutation associated with pediatric Cushing disease and other clinical features: a new syndrome. J Clin Endocrinol Metab. https://doi.org/10.1210/jc.2019-00697
Poncin J, Stevenaert A, Beckers A (1999) Somatic MEN1 gene mutation does not contribute significantly to sporadic pituitary tumorigenesis. Eur J Endocrinol 140 (6):573–576
Raitila A, Georgitsi M, Karhu A, Tuppurainen K, Makinen MJ, Birkenkamp-Demtroder K, Salmenkivi K, Orntoft TF, Arola J, Launonen V, Vahteristo P, Aaltonen LA (2007) No evidence of somatic aryl hydrocarbon receptor interacting protein mutations in sporadic endocrine neoplasia. Endocr Relat Cancer 14 (3):901–906. https://doi.org/10.1677/erc-07-0025
Tanizaki Y, Jin L, Scheithauer BW, Kovacs K, Roncaroli F, Lloyd RV (2007) P53 gene mutations in pituitary carcinomas. Endocr Pathol 18 (4):217–222. https://doi.org/10.1007/s12022-007-9006-y
Yagnik G, Jahangiri A, Chen R, Wagner JR, Aghi MK (2017) Role of a p53 polymorphism in the development of nonfunctional pituitary adenomas. Mol Cell Endocrinol 446:81–90. https://doi.org/10.1016/j.mce.2017.02.017
Gorvin CM, Newey PJ, Rogers A, Stokes V, Neville MJ, Lines KE, Ntali G, Lees P, Morrison PJ, Singhellakis PN, Malandrinou FC, Karavitaki N, Grossman AB, Karpe F, Thakker RV (2018) Association of prolactin receptor (PRLR) variants with prolactinomas. Hum Mol Genet. https://doi.org/10.1093/hmg/ddy396
Cai WY, Alexander JM, Hedley-Whyte ET, Scheithauer BW, Jameson JL, Zervas NT, Klibanski A (1994) ras mutations in human prolactinomas and pituitary carcinomas. J Clin Endocrinol Metab 78 (1):89–93. https://doi.org/10.1210/jcem.78.1.8288721
Trivellin G, Daly AF, Faucz FR, Yuan B, Rostomyan L, Larco DO, Schernthaner-Reiter MH, Szarek E, Leal LF, Caberg J-H, Castermans E, Villa C, Dimopoulos A, Chittiboina P, Xekouki P, Shah N, Metzger D, Lysy P, Ferrante E, Strebkova N, Mazerkina N, Zatelli M, Lodish M, Horvath A, de Alexandre R, Manning A, Levy I, Keil M, Sierra Mde L, Palmeira L, Coppieters W, Georges M, Naves L, Jamar M, Bours V, Wu T, Choong C, Bertherat J, Chanson P, Kamenický P, Farrell W, Barlier A, Quezado M, Bjelobaba I, Stojilkovic S, Wess J, Costanzi S, Liu P, Lupski J, Beckers A, Stratakis C (2014) Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med 371 (25):2363–2374
Assié G, Libé R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, Barreau O, Lefèvre L, Sibony M, Guignat L, Rodriguez S, Perlemoine K, René-Corail F, Letourneur F, Trabulsi B, Poussier A, Chabbert-Buffet N, Borson-Chazot F, Groussin L, Bertagna X, Stratakis C, Ragazzon B, Bertherat J (2013) ARMC5 mutations in macronodular adrenal hyperplasia with Cushing's syndrome. N Engl J Med 369 (22):2105–2114
Wang F, Gao H, Li C, Bai J, Lu R, Cao L, Wu Y, Hong L, Wu Y, Lan X, Zhang Y (2014) Low levels of PRB3 mRNA are associated with dopamine-agonist resistance and tumor recurrence in prolactinomas. J Neurooncol 116 (1):83–88. https://doi.org/10.1007/s11060-013-1276-2
Gao H, Wang F, Lan X, Li C, Feng J, Bai J, Cao L, Gui S, Hong L, Zhang Y (2015) Lower PRDM2 expression is associated with dopamine-agonist resistance and tumor recurrence in prolactinomas. BMC Cancer 15:272. https://doi.org/10.1186/s12885-015-1267-0
Bi WL, Horowitz P, Greenwald NF, Abedalthagafi M, Agarwalla PK, Gibson WJ, Mei Y, Schumacher SE, Ben-David U, Chevalier A, Carter S, Tiao G, Brastianos PK, Ligon AH, Ducar M, MacConaill L, Laws ER, Jr., Santagata S, Beroukhim R, Dunn IF (2017) Landscape of Genomic Alterations in Pituitary Adenomas. Clin Cancer Res 23 (7):1841–1851. https://doi.org/10.1158/1078-0432.ccr-16-0790
Wierinckx A, Delgrange E, Bertolino P, François P, Chanson P, Jouanneau E, Lachuer J, Trouillas J, Raverot G (2018) Sex-Related Differences in Lactotroph Tumor Aggressiveness Are Associated With a Specific Gene-Expression Signature and Genome Instability. Front Endocrinol (Lausanne) 9:706–706. https://doi.org/10.3389/fendo.2018.00706
De Sousa SM, McCabe MJ, Wu K, Roscioli T, Gayevskiy V, Brook K, Rawlings L, Scott HS, Thompson TJ, Earls P, Gill AJ, Cowley MJ, Dinger ME, McCormack AI (2017) Germline variants in familial pituitary tumour syndrome genes are common in young patients and families with additional endocrine tumours. Eur J Endocrinol 176 (5):635–644. https://doi.org/10.1530/eje-16-0944
Zhang Q, Peng C, Song J, Zhang Y, Chen J, Song Z, Shou X, Ma Z, Peng H, Jian X, He W, Ye Z, Li Z, Wang Y, Ye H, Zhang Z, Shen M, Tang F, Chen H, Shi Z, Chen C, Chen Z, Shen Y, Wang Y, Lu S, Zhang J, Li Y, Li S, Mao Y, Zhou L, Yan H, Shi Y, Huang C, Zhao Y (2017) Germline Mutations in CDH23, Encoding Cadherin-Related 23, Are Associated with Both Familial and Sporadic Pituitary Adenomas. Am J Hum Genet 100 (5):817–823. https://doi.org/10.1016/j.ajhg.2017.03.011
Rostomyan L, Daly AF, Petrossians P, Nachev E, Lila AR, Lecoq AL, Lecumberri B, Trivellin G, Salvatori R, Moraitis AG, Holdaway I, Kranenburg-van Klaveren DJ, Chiara Zatelli M, Palacios N, Nozieres C, Zacharin M, Ebeling T, Ojaniemi M, Rozhinskaya L, Verrua E, Jaffrain-Rea ML, Filipponi S, Gusakova D, Pronin V, Bertherat J, Belaya Z, Ilovayskaya I, Sahnoun-Fathallah M, Sievers C, Stalla GK, Castermans E, Caberg JH, Sorkina E, Auriemma RS, Mittal S, Kareva M, Lysy PA, Emy P, De Menis E, Choong CS, Mantovani G, Bours V, De Herder W, Brue T, Barlier A, Neggers SJ, Zacharieva S, Chanson P, Shah NS, Stratakis CA, Naves LA, Beckers A (2015) Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer 22 (5):745–757. https://doi.org/10.1530/erc-15-0320
Pepe S, Korbonits M, Iacovazzo D (2019) Germline and mosaic mutations causing pituitary tumours: genetic and molecular aspects. J Endocrinol 240 (2):R21-r45. https://doi.org/10.1530/joe-18-0446
De Sousa SMC, McCormack AI (2019) Aggressive Pituitary Tumors and Pituitary Carcinomas. In: Feingold KR, Anawalt B, Boyce A et al. (eds) Endotext. MDText.com, Inc., South Dartmouth (MA),
Newey PJ, Nesbit MA, Rimmer AJ, Head RA, Gorvin CM, Attar M, Gregory L, Wass JA, Buck D, Karavitaki N, Grossman AB, McVean G, Ansorge O, Thakker RV (2013) Whole-exome sequencing studies of nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 98 (4):E796–E800. https://doi.org/10.1210/jc.2012-4028
Sapkota S, Horiguchi K, Tosaka M, Yamada S, Yamada M (2017) Whole-Exome Sequencing Study of Thyrotropin-Secreting Pituitary Adenomas. J Clin Endocrinol Metab 102 (2):566–575. https://doi.org/10.1210/jc.2016-2261
Evans CO, Moreno CS, Zhan X, McCabe MT, Vertino PM, Desiderio DM, Oyesiku NM (2008) Molecular pathogenesis of human prolactinomas identified by gene expression profiling, RT-qPCR, and proteomic analyses. Pituitary 11 (3):231–245. https://doi.org/10.1007/s11102-007-0082-2
Wu ZB, Zheng WM, Su ZP, Chen Y, Wu JS, Wang CD, Lin C, Zeng YJ, Zhuge QC (2010) Expression of D2RmRNA isoforms and ERmRNA isoforms in prolactinomas: correlation with the response to bromocriptine and with tumor biological behavior. J Neurooncol 99 (1):25–32. https://doi.org/10.1007/s11060-009-0107-y
Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, Allen RG, Hnasko R, Ben-Jonathan N, Grandy DK, Low MJ (1997) Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron 19 (1):103–113
Brancati F, Camerota L, Colao E, Vega-Warner V, Zhao X, Zhang R, Bottillo I (2018) Biallelic variants in the ciliary gene TMEM67 cause RHYNS syndrome. Eur J Hum Genet 26 (9):1266–1271. https://doi.org/10.1038/s41431-018-0183-6
Wu Y, Berends MJ, Sijmons RH, Mensink RG, Verlind E, Kooi KA, van der Sluis T, Kempinga C, van dDer Zee AG, Hollema H, Buys CH, Kleibeuker JH, Hofstra RM (2001) A role for MLH3 in hereditary nonpolyposis colorectal cancer. Nat Genet 29 (2):137–138. https://doi.org/10.1038/ng1001-137
Bengtsson D, Joost P, Aravidis C, Askmalm Stenmark M, Backman AS, Melin B, von Salome J, Zagoras T, Gebre-Medhin S, Burman P (2017) Corticotroph Pituitary Carcinoma in a Patient With Lynch Syndrome (LS) and Pituitary Tumors in a Nationwide LS Cohort. J Clin Endocrinol Metab 102 (11):3928–3932. https://doi.org/10.1210/jc.2017-01401
Branford S, Wang P, Yeung DT, Thomson D, Purins A, Wadham C, Shahrin NH, Marum JE, Nataren N, Parker WT, Geoghegan J, Feng J, Shanmuganathan N, Mueller MC, Dietz C, Stangl D, Donaldson Z, Altamura H, Georgievski J, Braley J, Brown A, Hahn C, Walker I, Kim SH, Choi SY, Park SH, Kim DW, White DL, Yong ASM, Ross DM, Scott HS, Schreiber AW, Hughes TP (2018) Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood 132 (9):948–961. https://doi.org/10.1182/blood-2018-02-832253
De Sousa SM, Hardy TS, Scott HS, Torpy DJ (2018) Genetic Testing in Endocrinology. Clin Biochem Rev 39 (1):17–28
Thrane K, Eriksson H, Maaskola J, Hansson J, Lundeberg J (2018) Spatially Resolved Transcriptomics Enables Dissection of Genetic Heterogeneity in Stage III Cutaneous Malignant Melanoma. Cancer Res 78 (20):5970–5979. https://doi.org/10.1158/0008-5472.can-18-0747
Funding
SMCD is supported by an A.R. Clarkson Scholarship from the Royal Adelaide Hospital. This study was produced with support from a Royal Adelaide Hospital Health Services Charitable Gifts Board grant, and with the financial and other support of the Cancer Council SA’s Beat Cancer Project on behalf of its donors and the State Government of South Australia through the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional research committees (Melbourne Health: HREC/16/MH/132; Royal Adelaide Hospital: SSA/18/CALHN/445) and with the National Health and Medical Research Council guidelines.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Sousa, S.M.C., Wang, P.P.S., Santoreneos, S. et al. The Genomic Landscape of Sporadic Prolactinomas. Endocr Pathol 30, 318–328 (2019). https://doi.org/10.1007/s12022-019-09587-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-019-09587-0