Abstract
Background
Cerebrovascular autoregulation (CA) is a protective mechanism that enables the cerebral vasculature to automodulate tone in response to changes in cerebral perfusion pressure to ensure constant levels of cerebral blood flow (CBF) and oxygen delivery. CA can be impaired after neurological injury and contributes to secondary brain injury. In this study, we report novel impedance indices using trans-ocular brain impedance (TOBI) during controlled systemic hemorrhage and hypotension to assess CA in comparison with pressure reactivity index (PRx).
Methods
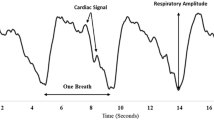
Yorkshire swine were instrumented to record intracranial pressure (ICP), mean arterial pressure (MAP), and CBF. TOBI was recorded using electrocardiographic electrodes placed on the closed eyelids. Impedance changes (dz) were recorded in response to introducing an alternating current (0.4 mA) through the electrodes. MAP, ICP, and CBF were also measured. Animals were subjected to a controlled hemorrhage to remove 30–40% of each animal’s total blood volume over 25–35 min. Hemorrhage was titrated to reach an MAP of approximately 35 mm Hg and end-tidal carbon dioxide above 28 mm Hg. PRx was calculated as a moving Pearson correlation between MAP and ICP. TOBI indices were calculated as the amplitude of the respiratory-induced changes in dz. DZx was calculated as a moving Pearson correlation between dz and MAP. TOBI indices (dz and DZx) were compared with hemodynamic indicators and PRx.
Results
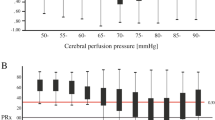
dz was shown to be highly correlated with MAP, ICP, cerebral perfusion pressure, and CBF (r = − 0.823, − 0.723, − 0.813, and − 0.726), respectively (p < 0.0001). During hemorrhage, cerebral perfusion pressure and CBF had a mean percent decrease (standard deviation) from baseline of − 54.2% (12.5%) and − 28.3% (14.7%), respectively, whereas dz increased by 277% (268%). Receiver operator characteristics and precision-recall curves demonstrated high predictive performance of DZx when compared with PRx with an area under the curve above 0.82 and 0.89 for receiver operator characteristic and precision-recall curves, respectively, with high sensitivity and positive predictive power.
Conclusions
TOBI indices appear to track changes in PRx and hemodynamics that affect CA during hemorrhage-induced hypotension. TOBI may offer a suitable, less invasive surrogate to PRx for monitoring and assessing CA.




Similar content being viewed by others
References
Armstead WM. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol Clin. 2016;34:465–77.
Atkins ER, Brodie FG, Rafelt SE, Panerai RB, Robinson TG. Dynamic cerebral autoregulation is compromised acutely following mild ischaemic stroke but not transient ischaemic attack. Cerebrovasc Dis. 2010;29:228–35.
Budohoski KP, Czosnyka M, de Riva N, et al. The relationship between cerebral blood flow autoregulation and cerebrovascular pressure reactivity after traumatic brain injury. Neurosurgery. 2012;71:652–60 (discussion 60-1).
Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis. 2003;16:69–75.
Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–7 (discussion 7-9).
Jaeger M, Schuhmann MU, Soehle M, Nagel C, Meixensberger J. Continuous monitoring of cerebrovascular autoregulation after subarachnoid hemorrhage by brain tissue oxygen pressure reactivity and its relation to delayed cerebral infarction. Stroke. 2007;38:981–6.
Reinhard M, Rutsch S, Lambeck J, et al. Dynamic cerebral autoregulation associates with infarct size and outcome after ischemic stroke. Acta Neurol Scand. 2012;125:156–62.
Cardim D, Robba C, Bohdanowicz M, et al. Non-invasive monitoring of intracranial pressure using transcranial Doppler ultrasonography: is it possible? Neurocrit Care. 2016;25:473–91.
Vranken NPA, Weerwind PW, Sutedja NA, Severdija EE, Barenbrug PJC, Maessen JG. Cerebral oximetry and autoregulation during cardiopulmonary bypass: a review. J Extra Corpor Technol. 2017;49:182–91.
Steppan J, Hogue CW Jr. Cerebral and tissue oximetry. Best Pract Res Clin Anaesthesiol. 2014;28:429–39.
Tackla R, Hinzman JM, Foreman B, Magner M, Andaluz N, Hartings JA. Assessment of cerebrovascular autoregulation using regional cerebral blood flow in surgically managed brain trauma patients. Neurocrit Care. 2015;23:339–46.
Czosnyka M, Czosnyka Z, Smielewski P. Pressure reactivity index: journey through the past 20 years. Acta Neurochir (Wien). 2017;159:2063–5.
Ang BT, Wong J, Lee KK, Wang E, Ng I. Temporal changes in cerebral tissue oxygenation with cerebrovascular pressure reactivity in severe traumatic brain injury. J Neurol Neurosurg Psychiatry. 2007;78:298–302.
Depreitere B, Guiza F, Van den Berghe G, et al. Pressure autoregulation monitoring and cerebral perfusion pressure target recommendation in patients with severe traumatic brain injury based on minute-by-minute monitoring data. J Neurosurg. 2014;120:1451–7.
Lazaridis C, Smielewski P, Steiner LA, et al. Optimal cerebral perfusion pressure: are we ready for it? Neurol Res. 2013;35:138–48.
Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36:1627–32.
Tiba MH, McCracken BM, Ansari S, et al. Novel noninvasive method of cerebrovascular blood volume assessment using brain bioimpedance. J Neurotrauma. 2017;34:3089–96.
Bernstein DP. Impedance cardiography: pulsatile blood flow and the biophysical and electrodynamic basis for the stroke volume equations. J Electr Bioimp. 2010;1:2–17.
Visser KR. Electric properties of flowing blood and impedance cardiography. Ann Biomed Eng. 1989;17:463–73.
Tiba MH, McCracken BM, Leander DC, et al. Trans-ocular brain impedance index for assessment of cerebral autoregulation in a porcine model of cerebral hemodynamic perturbation. J Clin Monit Comput. 2020. https://doi.org/10.1007/s10877-020-00556-1.
Animals NRCUCftUotGftCaUoL. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academy Press; 2011.
Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke. 2008;39:2531–7.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Efron B. Better Bootstrap Confidence Intervals. J Am Stat Assoc. 1987;82:171–85.
Davis JJ, Goadrich MH. The relationship between precision-recall and ROC curves. In: Proceedings of the 23rd international conference on Machine learning; 2006. p. 233–240.
Saito T, Rehmsmeier M. The precision-recall plot is more informative than the ROC plot when evaluating binary classifiers on imbalanced datasets. PLoS One. 2015;10:e0118432.
Babu JP, Jindal GD, Bhuta AC, Parulkar GB. Impedance plethysmography: basic principles. J Postgrad Med. 1990;36:57–63.
Bhuta AC, Babu JP, Jindal GD, Parulkar GB. Technical aspects of impedance plethysmography. J Postgrad Med. 1990;36:64–70.
van den Brule JMD, van der Hoeven JG, Hoedemaekers CWE. Cerebral perfusion and cerebral autoregulation after cardiac arrest. Biomed Res Int. 2018;2018:4143636.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10.
Rivera-Lara L, Zorrilla-Vaca A, Geocadin R, et al. Predictors of Outcome With Cerebral Autoregulation Monitoring: A Systematic Review and Meta-Analysis. Crit Care Med. 2017;45:695–704.
Tavakoli S, Peitz G, Ares W, Hafeez S, Grandhi R. Complications of invasive intracranial pressure monitoring devices in neurocritical care. Neurosurg Focus. 2017;43:E6.
Caldas JR, Haunton VJ, Panerai RB, Hajjar LA, Robinson TG. Cerebral autoregulation in cardiopulmonary bypass surgery: a systematic review. Interact Cardiovasc Thorac Surg. 2018;26:494–503.
Choi S, Avramescu S, Orser BA, Au S. Protocol for a prospective cohort study of assessing postoperative cognitive changes after total hip and knee arthroplasty in the Greater Toronto area. BMJ Open. 2019;9:e024259.
Donnelly J, Czosnyka M, Adams H, et al. Individualizing thresholds of cerebral perfusion pressure using estimated limits of autoregulation. Crit Care Med. 2017;45:1464–71.
Inouye SK. Delirium in older persons. N Engl J Med. 2006;354:1157–65.
Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30.
Sekhon MS, Gooderham P, Menon DK, et al. The burden of brain hypoxia and optimal mean arterial pressure in patients with hypoxic ischemic brain injury after cardiac arrest. Crit Care Med. 2019;47:960–9.
Acknowledgements
The authors would like to thank Katelyn Murphy and the Michigan Center for Integrative Research in Critical Care for their technical support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by MT, BM, DL, CCM, and NG. The first draft of the manuscript was written by MT, BM and KW. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Source of Support
This study was supported by a grant from the Department of Defense (#W81XWH-18-1-0005).
Conflict of interest
Dr. Tiba and Dr. Ward report grants from Department of Defense, during the conduct of the study; In addition, Dr. Tiba and Dr. Ward have a patent 16/613,707 pending which is assigned to the University of Michigan. The technology has been licensed from the University of Michigan to New Vital Signs, Inc. Dr. Ward has equity in the company. Dr. Williamson reports grants from Department of Defense, during the conduct of the study. Dr. Picton, Mr. McCracken, Ms. Leander, Ms. Colmenero Mahmood, and Mr. Greer have nothing to disclose.
Ethical approval
All procedures outlined in this study adhere to the principles stated in the eighth edition of the Guide for the Care and Use of Laboratory Animals and were approved by the University of Michigan’s Institutional Animal Care and Use Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tiba, M.H., McCracken, B.M., Leander, D.C. et al. Trans-Ocular Brain Impedance Indices Predict Pressure Reactivity Index Changes in a Porcine Model of Hypotension and Cerebral Autoregulation Perturbation. Neurocrit Care 36, 139–147 (2022). https://doi.org/10.1007/s12028-021-01272-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-021-01272-7