Abstract
Ferroptosis is a recently defined form of cell death with the involvement of iron and reactive oxygen species (ROS), which is distinct from apoptosis, autophagy and other forms of cell death. Emerging evidence suggested that iron accumulation and lipid peroxidation can be discovered in various neurological diseases, accompanied with reduction of glutathione (GSH) and glutathione peroxidase 4 (GPX4). In addition, ferroptotic inhibitors have been shown to protect neurons, and recover the cognitive function in disease animal models. This review summarizes the mechanisms underlying ferroptosis and reviews the contributions of ferroptosis in neurodegenerative diseases (i.e. Alzheimer’s disease and Parkinson’s disease), traumatic brain injury, as well as hemorrhagic and ischemic stroke, to provide the current understanding of this novel form of cell death in neurological disorders.
Similar content being viewed by others
Introduction
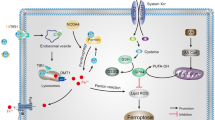
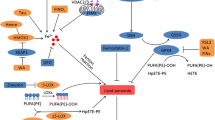
Ferroptosis is an iron-dependent form of regulated cell death which is distinct from apoptosis, classic necrosis, autophagy and other forms of cell death (Dixon et al. 2012). It is characterized morphologically by the vanishing of mitochondria membranes. Increasing evidence indicates that ferroptosis is associated with reduced detoxification of lipid peroxides by glutathione peroxidase 4 (GPX4). In addition, peroxidation of polyunsaturated fatty acids (PUFAs) was also considered a key driver of ferroptosis (Kagan et al. 2017). Oxidation of PUFAs by lipoxygenases (LOX) leads to the accumulation of peroxides that may contribute to the generation of lipid peroxide breakdown products (Yang et al. 2016), and consequently results in the release of lipid death signals that propagate ferroptotic cell death. The current understanding of ferroptosis pathways is illustrated in Fig. 1.
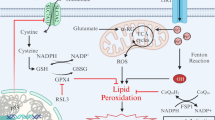
Molecular pathways involved in ferroptosis. The mechanism summary of occurrence and regulatory function of ferroptosis (Stockwell et al. 2017). Transferrin receptor 1 (TfR1) binds transferrin together to form a complex for ferric iron (Fe3+) uptake. Because of the ferrireductase activity of STEAP3, Fe3+ is reduced to ferrous iron (Fe2+) in the endosome. Then, divalent metal transporter 1 (DMT1) translates Fe2+ to a labile iron pool from endosome. Maintenance of iron homeostasis is dependent on the ferroportin and ferritin. Fe2+ export is mediated by ferroportin that is an iron efflux pump on the cell membrane. Ferritin is a protein for excess iron storage. On the one hand, Fe2+ produces lipid ROS through Fenton reaction, and on the other hand, it catalyzes lipid peroxidation by combining with LOXs (Shintoku et al. 2017). Iron chelators like DFO, CPX and 2,2-BP can inhibit ferroptosis (Barradas et al. 1989; Abeysinghe et al. 1996). Lipoxygenase inhibitors such Baicalein, Vitamin E, ML351 and Zileuton can depress LOXs activity to rescue cells from ferroptosis (Kagan et al. 2017; Tuo et al. 2017). Fer-1 and Lip-1 inhibit radical-trapping antioxidants (RTAs) which activate LOXs to prevent ferroptosis in cells (Dixon et al. 2012). A class of ferroptosis inducers such as erastin, glutamate, and sulfasalazaine reduce the concentration of intracellular cystine by inhibiting system xc-, which leads to a decline in GSH content (Stockwell et al. 2017). GPX4 can be combined with GSH to alcoholize lipid peroxides in cells, and then prevent the occurrence of ferroptosis (Gaschler et al. 2018). Another type of ferroptosis inducer RSL3 inhibits activity of GPX4 to reduce the alcoholization of lipid peroxides which cause ferroptosis (Dixon et al. 2012; Gaschler et al. 2018)
Recent discoveries have revealed a close connection between ferroptosis and neurological disorders, including neurodegenerative diseases and brain damage. Inhibitors of ferroptosis, such as ferrostatins-1 and liproxstatins-1, are protective in models of degenerative brain disorders, including Parkinson’s disease, as well as ischemic and hemorrhagic stroke (Do Van et al. 2016; Li et al. 2017; Tuo et al. 2017; Zille et al. 2017). Therefore, the optimization of existing inhibitors or the development of novel inhibitors of ferroptosis could be a potential approach for these neurological diseases.
Role of Iron in Ferroptosis
Iron is essential for the execution of ferroptosis, since iron chelators such as deferoxamine (DFO), ciclopirox (CPX), and 2,2-bipyridyl (2,2-BP) can rescue erastin- and RSL3-induced ferroptosis (Yang and Stockwell 2008; Dixon et al. 2012). Moreover, ferroptosis induced by erastin or RSL3 is prevented by shRNA knockdown of TFRC, which encodes the transferrin receptor protein 1 (TfR1) required for iron import (Yang and Stockwell 2008; Gao et al. 2015). Conversely, adding iron-bound transferrin or a bioavailable form of iron (e.g., ferric ammonium citrate, ferric citrate, iron chloride hexahydrate), but not other divalent transition metal ions (such as Cu2+, Mn2+, Ni2+, and Co2+), potentiated erastin-induced ferroptosis (Dixon et al. 2012; Gao et al. 2015).
How iron promotes ferroptosis inside the cell remains unclear. Generally it is believed that iron promotes ferroptosis by donating electrons to oxygen to form ROS (Stockwell et al. 2017). On the other hand, iron is also an important component of the catalytic subunit of LOX. The iron-dependent LOX enzymes catalyze site-specific oxidation of PUFAs such as arachidonic acid, and are directly inactivated by lipophilic iron chelators (e.g. CPX, 2,2-BP) (Barradas et al. 1989; Abeysinghe et al. 1996; Kuhn et al. 2015). DFO is a membrane impermeable iron chelator that accumulates in the lysosome through endocytosis (Barradas et al. 1989), suggesting that lysosomal iron may also be involved in ferroptosis.
Role of Glutathione in Ferroptosis
The uptake of cystine by glutamate/cysteine antiporter (system xc−), consisting of a 12-pass transmembrane protein transporter solute carrier family 7 member 11 (SLC7A11) and a single-pass transmembrane regulatory protein solute carrier family 3 member 2 (SLC3A2), was inhibited in erastin-induced ferroptosis (Dixon et al. 2012). Therefore, inhibition of system xc- results in the depletion of the intracellular cysteine pool (Dixon et al. 2012). Cysteine plays a significant roles in cells, and in the context of ferroptosis it acts as a building block for the biosynthesis of glutathione (GSH).
GSH is required for the lipid repair function of GPX4 as its substrate. Depletion of GSH through cysteine starvation leads to loss of GPX4 activity, resulting in accumulation of unrepaired lipid peroxides and ferroptosis (Friedmann Angeli et al. 2014). GPX4 converts reduced GSH to oxidized glutathione (GSSG) to reduce lipid hydroperoxides to their corresponding alcohols or free hydrogen peroxide to water (Gaschler et al. 2018). Selenium (Se) is a key regulator of GPX4 activity. Selenium-containing wild-type GPX4 efficiently reduces peroxides to their corresponding alcohols, therefore preventing ferroptotic cell death (Ingold et al. 2018). On the other hand, a redox inactive selenium cysteine to serine substitution in GPX4 does not rescue early embryonic lethality as reported for GPX4−/− mice (Ingold et al. 2015), suggesting that its redox activity is essential to GPX4 cellular function.
GSH is also the natural ligand for Fe2+ in the labile iron pool (LIP), which is an exchangeable pool of loosely ligated iron within neurons (Hider and Kong 2011). GSH binds to Fe2+ in the LIP to prevent iron oxidation, which not only maintains solubility of Fe2+ (Fe3+ is highly insoluble), but also prevents Fe2+ acting as a catalyst for the production of the potent oxidant, the hydroxyl radical, from physiologically available hydrogen (Hider and Kong 2011). Thus, direct inhibition of GSH synthesis would trigger ferroptosis.
Role of Lipid Peroxidation in Ferroptosis
Lipid metabolism is also closely associated with ferroptosis. Nitrogen oxides (NOXs) provide an accumulation source of ROS in erastin-induced ferroptosis, which has been reported to adjust the sensitivity to erastin in tumor cells (Dixon et al. 2012). On the other hand, the production of cell membrane lipid peroxide is also the source of ROS, which drives ferroptosis. The abundance and location of polyunsaturated fatty acids (PUFAs) determine the degree of lipid peroxidation that occurs in the cell and thus the extent of the ferroptosis effect. The most susceptible lipid is polyunsaturated-fatty-acid-containing phospholipids (PUFA-PLs) which can drive the subsequent cell death (Doll et al. 2017). And free PUFAs need to be esterified to form membrane phospholipids and oxidized into ferroptotic signals to synthesize lipid signaling, especially phosphatidylethanolamine (PE)-containing phospholipids with arachidonate or adrenate moieties (Kagan et al. 2017). In membrane lipid environments, PUFAs are specifically peroxidized in ferroptosis (Doll et al. 2017; Kagan et al. 2017). There are three well-defined classes of lipid oxidation enzymes: cyclooxygenases (COXs), cytochrome p450 (CYPs), and LOXs, among which LOX enzymes have been found to be the most important for ferroptosis. LOXs are a family of nonheme, iron-containing enzymes that catalyze deoxygenation of PUFAs (Shintoku et al. 2017). The mechanism of LOXs in ferroptosis still remains elusive; yet several LOX inhibitors can protect MEFs and pancreatic cancer cells from erastin (Shintoku et al. 2017).
Some genes involving the synthesis of fatty acids ensure sufficient membrane lipid PUFA production to facilitate ferroptosis for subsequent lipid peroxidation and ROS production (Kagan et al. 2017). Enzymes encoded by corresponding genes such as acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) are involved in the biosynthesis and remodeling of polyunsaturated fatty acid-PEs in cellular membranes, preventing the ferroptosis induced by the inhibition of GPX4 (Doll et al. 2017). Deletion of ACSL4 and LPCAT3 results in resistance to ferroptosis in RSL3 and ML162-induced KBM7 cells or in mammals (Stockwell et al. 2017).
Ferroptosis in Neurological Disorders
Ferroptosis, as a way to promote cell death, may be implicated in a number of diseases, including cancer and neurological disorders. Several reviews already provided comprehensive summaries to place ferroptosis as one of the major cell death forms involved in disease (e.g. Stockwell et al. 2017). Based on the ‘Metal Theory’ for neurodegeneration (Ayton et al. 2013), a couple of groups recently have tested the hypothesis that ferroptosis may be the cell death form of neurons during the disease progression, and the relevant evidence is summarized in Table 1.
Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common neurodegenerative disease characterized by progressive cognitive impairment as well as pathological plaques and tangles in the brain. However, there is no effective means to delay the progression of the disease nor to prevent the occurrence of disease despite over 100 years of research since its discovery, and it is still under debate about the importance of these pathological hallmarks in disease. On the other hand, dyshomeostasis of metals such as iron have been suggested to be responsible for the neuronal death in the disease, triggered by the formation of tangles and plaques. The brain MRI scans of AD patients revealed elevated iron in the areas of the brain affected by AD, such as severely damaged hippocampus (Raven et al. 2013). Accumulation of iron in hippocampus and cerebral cortex is co-localized with typical AD pathology (Ayton et al. 2015a), and iron can advance the accumulation and/or aggregation of the Aβ and tau (Adeghate and Parvez 2000; Yamamoto et al. 2002). Amyloid precursor protein (APP) and tau collaboratively facilitate the export of iron, and their deficits in AD were suggested responsible for iron accumulation in AD (Duce et al. 2010; Lei et al. 2012, 2017; Li et al. 2015).
Iron accumulation induces the ROS production in the brain of AD, which is significantly higher than in healthy control brains (Ayton et al. 2015b). Oxidative stress facilities the neurotoxic oligomerization process of Aβ and tau tangles (Lane et al. 2018). The neuronal death involved in the process can now be explained by ferroptosis. The guanine-rich RNA sequence binding factor 1 (GRSF1), which controls the translation of GPX4, was downregulated in Alzheimer’s disease mice (Yoo et al. 2010). Knocking out GPX4 in mice directly resulted in age-dependent neurodegenerative changes and significant neuronal loss, which was obviously worsened after feeding a vitamin E deficient diet (Hambright et al. 2017). Both GPX4 and vitamin E are endogenous ferroptosis inhibitors, and the fact that ablation of GPX4 in forebrain neurons resulted in cognitive impairment and hippocampal neurodegeneration in Gpx4 brain inducible knockout (Gpx4BIKO) mice, is consistent with AD pathology (Hambright et al. 2017). Moreover, in vitro studies have found that GSH depletion increased neuronal death in neurons isolated from aged 3xTg-AD neurons (Ghosh et al. 2014). Se, a key regulator of GPX4 activity, is crucial to the brain but it may be potentially neurotoxic, depending on dosage and speciation; and Se deficiency is considered to be related to cognitive impairment and AD pathologies (Chmatalova et al. 2017; Vinceti et al. 2017).
Therapeutically, iron chelator has already trialed in AD previously (Crapper McLachlan et al. 1991), and a randomized, multi-center, double-blind, placebo-controlled Phase IIa trial using deferiprone for AD is currently ongoing in Melbourne (Deferiprone to Delay Dementia, the 3D study). In mice, means to regulate ferroptosis were tested for AD. For example, α-Lipoic acid (LA) (a natural coenzyme factor as antioxidants and iron chelators) was shown to block P38 and ensure normal expression of GPX4, therefore counteract neurotoxicity and cognitive dysfunction, and mice behavioral tests demonstrated that spatial memory and cognitive ability of LA-treated P301S tau transgenic mice was significantly better than control-feed mice (Zhang et al. 2018). Furthermore, GSH content increases after N-acetylcysteine (NAC) [NAC (200 mg/kg) was intraperitoneally (ip) injected to mice for 7 days before testing] treatment while lipid oxidation decreases in mice when intracerebroventricularly injected with the aggregated amyloid β-peptide to produce AD phenotype (Fu et al. 2006). Supplementation with two organic forms of Se, Se-enriched yeast (Se-yeast) and selenomethionine (Se-Met), could improve cognitive impairment, reverse synaptic deficits and mitigate tau pathology in triple-transgenic (3× Tg) AD mice (Zhang et al. 2017, 2018).
Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disease. Similar to AD, the most effective treatment on the market for PD, L-dopa supplementation therapy, is only symptomatic. The dominant pathological feature of PD is the dopaminergic neuronal degeneration in substantia nigra pars compacta (SNpc) where iron is particularly abundant (Ayton and Lei 2014) as critical participants of tyrosine hydroxylase-dependent dopamine synthesis and other dopamine metabolism processes (Do Van et al. 2016; Belarbi et al. 2017). α-synuclein (α-syn) also has a IRE which implies a potential role in iron regulation (Duce et al. 2017; Zhou and Tan 2017). In the presence of copper catalyst, α-synuclein (α-syn) has the potential of ferrireductase, which when combined with Fe3+ and converted to Fe2+, incorporate with C-terminal of α-syn (Davies et al. 2011). Iron also increases the rate of α-syn fibril formation, a major event in PD (Abeyawardhane et al. 2018). The iron chelator DFP, which reduces oxidative stress and increases dopamine activity to improve existing motor neurological symptoms and reduce deterioration of motor function, has already been found to have neuroprotective effects in patients with early PD (Do Van et al. 2016).
MPP + −induced SH-SY5Y (a frequently used Parkinson’s disease model) cell line, which is not a cell programmed death, has some similarities to ferroptosis: both involve lipid peroxidation and can be inhibited by DIM and Fer-1 (a type of radical-trapping antioxidants). It was shown that iron chelators not only have an inhibitory effect on ferroptosis, but also protect dopamine neurons from cell death (Ayton and Lei 2014). In addition, the study found that GSH reduced in MPTP mouse model (Feng et al. 2014), and GSH depletion potentiates MPP+ toxicity in nigral dopaminergic neurons (Wullner et al. 1996). These studies suggested that ferroptosis is involved in the dopamine neuron degeneration of PD.
Huntington’s Disease
Huntington’s disease (HD) is a progressive neurodegenerative disease characterized by rapid involuntary movements and cognitive impairment that ultimately leads to death, which is caused by expansion of CAG repeats in the huntingtin (HTT) gene. HD is pathologically featured by iron accumulation as well as abnormal levels of glutamate and glutathione (Skouta et al. 2014; Agrawal et al. 2018). It was also reported that HD patients have lower GSH content in plasma samples (Klepac et al. 2007), and lower GPX activity in erythrocyte, linking ferroptosis with HD. A HD mouse model, with mice treated with nitropropionic acid, also showed a reduction in overall (cytoplasmic and mitochondrial) GSH reduction and inhibited glutathione S-transferase (GST) function in hippocampus and cortex, accompanied by its HD phenotype (Klivenyi et al. 2000).
Although the underlying mechanism for the neurodegeneration caused by the mutant huntingtin protein remains unclear, the ability of huntingtin to induce oxidative damage has been demonstrated. One study found that Fer-1 treated at 10 nM, 100 nM, and 1 μM protects neurons which are marked with yellow fluorescent protein (YFP), and induced the cell death by expression, via biolistic transfection, of a huntingtin (htt) exon 1 fragment with a pathogenic repeat (73Q) (mN90Q73). And the number of medium spiny neurons (MSNs) was significantly increased compared with the control group (Skouta et al. 2014). Besides, iron chelator DFO provides a protective effect in R6/2 mice (HD mice model) (Yang et al. 2016).
Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease which affects motor neurons in cortex, spinal cord and brainstem, and its clinical presentations include progressive atrophy and weakness of the limbs and trunk muscles. The mechanism of ALS remains unidentified, and cure of the disease is not yet available. Of note, spinal cord iron accumulation has been reported in mouse models of ALS, mutant SOD1G37R, SOD1G86R and SOD1G93A mice (Golko-Perez et al. 2017). In human ALS, high-resolution MRI revealed iron accumulation in motor cortex in vivo and in autoptic brain, in the latter confirmed by iron histochemistry (Kwan et al. 2012). Besides, lipid peroxidation was significantly increased in the erythrocytes of ALS patients while GSH content was lower than control (Babu et al. 2008; Johnson et al. 2012), and similar results also appeared in ALS mice (Mathews and Leiter 1999). It was also reported that depletion of GSH promotes the motor neuron degeneration in ALS. Furthermore, motor neurons in ALS are sensitive to GPX4 knockout-induced cell death (Conrad et al. 2018), suggesting a direct involvement of ferroptosis in ALS pathogenesis.
Friedreich’s Ataxia
Friedreich’s Ataxia (FRDA) is an autosomal recessive neurodegenerative disease characterized by ataxia, areflexia and loss of vibratory and position sense. The most common cause of FRDA is trinucleotide amplification of GAA in the gene encoding frataxin, which is associated with iron accumulation in mitochondria. Besides, FRAD neurons showed higher LIP, increased ROS and lower reduced GSH levels, and enhanced sensitivity to oxidants compared with control neurons (Koeppen et al. 2011; Codazzi et al. 2016). Analysis of a yeast FRDA model lacking the fragment of frataxin showed a marked increase in GPX4 activity with reduction in NADPH levels, which therefore limited the ability of glutathione reductase (GR) to recycle GSSG to GSH (Auchere et al. 2008; Johnson et al. 2012). Furthermore, there are preliminary experiments suggesting that patients with FRDA suffer a disturbance of GSH homeostasis and modifications of GSH-dependent antioxidant (Napoli et al. 2006). These results indicate that ferroptosis at least casually links with FRDA, and further research to support such hypothesis is needed.
Traumatic Brain Injury
Traumatic brain injury (TBI) is acknowledged as high mortality rate and complicated situations of patients who survive the injury. These complications result in the economic burden of society and influence the quality of life of the individuals and families. Iron is considered to contribute to various pathways of secondary injury after brain trauma, including oxidative stress and inflammation (Ayton et al. 2014; Ma et al. 2017). Studies indicated that iron chelators such as DFO improved cognitive function after TBI (Khalaf et al. 2018). Another study showed remarkably enhanced generation of lipid peroxidation products and depletion of GSH and ascorbate after experimental TBI in rats (Bayir et al. 2002). There are also studies which showed that GSH decreased in head trauma model mice (Di Pietro et al. 2014), and GPx activity decreased after TBI (Xu et al. 2014). Demonstration of the elevated levels of 15-HpETE-PE, which contribute to ferroptosis in the brain cortex and hippocampus after TBI, accompanied by increased expression of 15LO2 (a catalyzer of the formation of pro-ferroptotic 15-OOH-eicosatetraenoic) and consumption of GPX4 effectively indicated the possibility of ferroptotic death in TBI (Wenzel et al. 2017).
Established ferroptosis inhibitors have not been tested in the treatment of traumatic brain injury, yet studies discovered that the N,N′-Di (2-hydroxybenzyl) ethylenediamine-N,N′-diacetic acid monohydrochloride (HBED) could be a therapeutic tool as iron chelator to facilitate the recovery process after TBI (Khalaf et al. 2018). Depressing the ability of PEBP1/15LO complex to synthesize 15-HpETE-PE could provide a new way to anti-ferroptosis after TBI (Wenzel et al. 2017).
Stroke
Stroke is the second leading cause of mortality worldwide and is the most common cause of long-term disability including dementia, and its incidence is expected to rise with the projected increase in the aging population. Ischemic stroke comprises 80% of all stroke events; however, only tissue plasminogen activator (tPA) was approved by FDA for ischemic stroke treatment. tPA needs to be applied within three hours of the onset of ischemic stroke (National Institute of Neurological and Stroke rt. 1995), but these patients account for less than 5% of the patients with ischemic stroke. Therefore, additional stroke therapies are highly desirable. Disturbance of brain iron homeostases have been linked to acute neuronal injury following ischemic stroke (Kondo et al. 1995). Iron is suggested to induce neuronal injury by catalyzing a sequence of Fenton’s Chemistry known as Haber-Weiss reaction, in which superoxide and hydrogen peroxide (H2O2) are converted into highly reactive toxic hydroxyl radicals (OH·) (Kehrer 2000). Magnetic resonance imaging (MRI) in children following severe ischemic-hypoxic insult and subsequent resuscitation showed areas of increased iron deposition in the basal ganglia, thalami, and periventricular and subcortical white matter (Dietrich and Bradley 1988). Results obtained from mammalian experimental research support a pathogenic relationship between iron and ischemic neuronal injury (Kondo et al. 1995; Tuo et al. 2017). Iron chelators (such as DFO) were shown to improve outcome after ischemic stroke in mammals (Van Hoecke et al. 2005; Hanson et al. 2009). In addition, brain iron elevation occurs with normal human aging (Ayton et al. 2013), coinciding with aging being the most significant risk factor for ischemic stroke.
However, the mechanism of iron-related neurotoxicity in ischemic stroke is yet to be investigated. The latest research found that iron may lead to neuronal death via ferroptosis in an animal model of ischemic stroke (Tuo et al. 2017). Inhibitors of ferroptosis, such as ferrostatin-1 and liproxstatin-1, acted to protect from cerebral ischemic injury in a mouse model (Tuo et al. 2017). There have been studies showing the level of GSH was markedly reduced and lipid peroxidation increased in a mouse model of ischemic brain stroke (Ahmad et al. 2014), and GSH-Px activity decreased significantly in PC12 cells during oxygen-glucose deprivation (OGD) (Liu et al. 2017). LOX-mediated generation of lipid hydroperoxides has been suggested to be involved in ferroptosis (Li et al. 2018), and has been shown to be increased in stroke (Yigitkanli et al. 2013). In line with these findings, several LOX inhibitors have been found to reduce infarct size in ischemic stroke (Yigitkanli et al. 2013; Tuo et al. 2017).
Although less common, hemorrhagic stroke is a disease with high fatality and morbidity. And similar to ischemic stroke, no effective therapies are available for hemorrhagic stroke. The adverse consequences of intracerebral hemorrhage (ICH) are mainly due to irreversible damage to neurons caused by primary and secondary injuries respectively. Among them, the occurrence of secondary injury is mainly due to hemoglobin and its oxidized product hemin from lysed erythrocyte. Clinical studies found that serum ferritin, an iron storage protein, was upregulated after ICH and was independently associated with severe brain edema and poor prognosis following ICH; the higher the serum ferritin levels, the poorer the outcome in patients with ICH (Mehdiratta et al. 2008; Perez de la Ossa et al. 2010).
Researchers found that cell death found in hemorrhagic stroke shares features of ferroptosis: (1) shrunken mitochondria, and (2) ferroptosis inhibitors can eliminate hemoglobin-induced cell death by inhibiting hemin toxicity. Intraventricular injection of Fer-1 in the ICH model significantly reduced ROS concentration and protected neurons from degeneration and reduced lesion volume. Fer-1 also suppressed expression of COX-2, which is over expressed in neurons after ICH. (3) Hemorrhagic stroke in vitro or in vivo shows molecular features of ferroptosis (Li et al. 2017). After 4 h of hemin treatment in vitro, researchers found increasing phospho-ERK1/2 that was involved in the activation of mitogen-activated protein (MAP) kinase signaling, which is crucial in erastin-induced ferroptosis in tumor cells carrying oncogenic Ras. As expected, phospho-ERK1/2 was also increased significantly after ICH in rats. Besides, four genes in tumor cells induced by ferroptosis (IREB2, ATP5G3, CS, RPLl8) were also induced in ICH mice (Li et al. 2017). These results suggest that ferroptosis is involved in the secondary injury process of ICH.
Ferroptosis inhibitors, especially DFO and N-acetylcysteine, provide obvious functional recovery in several models of ICH. Apart from ferroptosis inhibitors, abrogating the increase in RIP1 and RIP3 gene expression and phospho-RIP1 is a new research direction which is based on hypothesis that ferroptosis may induce an affector phase of death, leading to a necroptotic effector phase (Zille et al. 2017). In fact, the effect of combination therapy is better than that of ferroptosis inhibitor alone (Zille et al. 2017). These new findings provide new ideas for the treatment of hemorrhagic stroke.
Periventricular Leukomalacia
Periventricular leukomalacia (PVL) is the leading cause of neurological disabilities including motor and cognitive deficits in premature infants (Ceschin et al. 2015). The main pathological changes in PVL are generation of free radicals and white matter damage (Welin et al. 2007). Mechanistically, progenitor oligodendrocytes (OLs) were damaged in PVL, suppressing myelination and neuronal signaling, which resulted in white matter rarefaction and ultimately cerebral palsy and cognitive impairment (Brault et al. 2004). The causes of inherent vulnerability in progenitor OLs may include activation of glutamate receptors and cellular iron content, which indicate the potential role of ferroptosis. On the basis of MRI examinations in neonates and infants with perinatal asphyxia, results showed iron deposition in thalamus and basal ganglia in neonates and infants with severe perinatal asphyxia (Baenziger et al. 1993). In addition, elevated lipid ROS biomarkers were found at autopsy in children with PVL (Skouta et al. 2014). Analysis of patients’ cerebrospinal fluid suggested that there was an abundance of lipid peroxides such as 8-isoprostane and malondialdehyde (MDA). Ferroptosis inhibitor Fer-1 at 100 nM concentration completely protected OLs from cystine-free conditions which ultimately deplete glutathione (Skouta et al. 2014). Vitamin E, also a natural ferroptosis inhibitor, can prevent cell death induced by glutathione deletion in rat OL cultures (Yang et al. 2016). However, PVL rats exhibited reduced plasma and tissue GSH (Izzet et al. 2005).
Concluding Remarks
Neurological disorders, especially neurodegenerative disorders, impose a great burden on patients, patients’ families, and society. As summarized here, the discovery of ferroptosis and the follow-up studies on its potential involvement in diseases provide not only mechanistic insights into the neuronal loss, but also trackable targets for the diseases. With a significant proportion of details still to investigate, we have now begun to understand the molecular pathways involved in ferroptosis and to discover its inhibitors, and hopefully in the near future we would have drug candidates for testing in neurological disorders.
References
Abeyawardhane DL, Fernandez RD, Murgas CJ, Heitger DR, Forney AK, Crozier MK, Lucas HR (2018) Iron redox chemistry promotes antiparallel oligomerization of alpha-Synuclein. J Am Chem Soc 140(15):5028–5032
Abeysinghe RD, Roberts PJ, Cooper CE, MacLean KH, Hider RC, Porter JB (1996) The environment of the lipoxygenase iron binding site explored with novel hydroxypyridinone iron chelators. J Biol Chem 271(14):7965–7972
Adeghate E, Parvez SH (2000) Nitric oxide and neuronal and pancreatic beta cell death. Toxicology 153(1–3):143–156
Agrawal S, Fox J, Thyagarajan B, Fox JH (2018) Brain mitochondrial iron accumulates in Huntington’s disease, mediates mitochondrial dysfunction, and can be removed pharmacologically. Free Radic Biol Med 120:317–329
Ahmad S, Elsherbiny NM, Haque R, Khan MB, Ishrat T, Shah ZA, Khan MM, Ali M, Jamal A, Katare DP, Liou GI, Bhatia K (2014) Sesamin attenuates neurotoxicity in mouse model of ischemic brain stroke. Neurotoxicology 45:100–110
Auchere F, Santos R, Planamente S, Lesuisse E, Camadro JM (2008) Glutathione-dependent redox status of frataxin-deficient cells in a yeast model of Friedreich’s ataxia. Hum Mol Genet 17(18):2790–2802
Ayton S, Lei P (2014) Nigral iron elevation is an invariable feature of Parkinson’s disease and is a sufficient cause of neurodegeneration. Biomed Res Int 2014:581256
Ayton S, Lei P, Bush AI (2013) Metallostasis in Alzheimer’s disease. Free Radic Biol Med 62:76–89
Ayton S, Zhang M, Roberts BR, Lam LQ, Lind M, McLean C, Bush AI, Frugier T, Crack PJ, Duce JA (2014) Ceruloplasmin and beta-amyloid precursor protein confer neuroprotection in traumatic brain injury and lower neuronal iron. Free Radic Biol Med 69:331–337
Ayton S, Lei P, Hare DJ, Duce JA, George JL, Adlard PA, McLean C, Rogers JT, Cherny RA, Finkelstein DI, Bush AI (2015a) Parkinson’s disease iron deposition caused by nitric oxide-induced loss of beta-amyloid precursor protein. J Neurosci 35(8):3591–3597
Ayton S, Faux NG, Bush AI (2015b) Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat Commun 6:6760
Ayton S, Lei P, Bush AI (2015c) Biometals and their therapeutic implications in Alzheimer’s disease. Neurotherapeutics 12(1):109–120
Babu GN, Kumar A, Chandra R, Puri SK, Singh RL, Kalita J, Misra UK (2008) Oxidant-antioxidant imbalance in the erythrocytes of sporadic amyotrophic lateral sclerosis patients correlates with the progression of disease. Neurochem Int 52(6):1284–1289
Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ (1998) Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci 18(16):6241–6253
Baenziger O, Martin E, Steinlin M, Good M, Largo R, Burger R, Fanconi S, Duc G, Buchli R, Rumpel H et al (1993) Early pattern recognition in severe perinatal asphyxia: a prospective MRI study. Neuroradiology 35(6):437–442
Barradas MA, Jeremy JY, Kontoghiorghes GJ, Mikhailidis DP, Hoffbrand AV, Dandona P (1989) Iron chelators inhibit human platelet aggregation, thromboxane A2 synthesis and lipoxygenase activity. FEBS Lett 245(1–2):105–109
Bayir H, Kagan VE, Tyurina YY, Tyurin V, Ruppel RA, Adelson PD, Graham SH, Janesko K, Clark RS, Kochanek PM (2002) Assessment of antioxidant reserves and oxidative stress in cerebrospinal fluid after severe traumatic brain injury in infants and children. Pediatr Res 51(5):571–578
Belarbi K, Cuvelier E, Destee A, Gressier B, Chartier-Harlin MC (2017) NADPH oxidases in Parkinson’s disease: a systematic review. Mol Neurodegener 12(1):84
Brault S, Martinez-Bermudez AK, Roberts J 2nd, Cui QL, Fragoso G, Hemdan S, Liu HN, Gobeil F Jr, Quiniou C, Kermorvant-Duchemin E, Lachance C, Almazan G, Varma DR, Chemtob S (2004) Cytotoxicity of the E(2)-isoprostane 15-E(2t)-IsoP on oligodendrocyte progenitors. Free Radic Biol Med 37(3):358–366
Ceschin R, Lee VK, Schmithorst V, Panigrahy A (2015) Regional vulnerability of longitudinal cortical association connectivity: associated with structural network topology alterations in preterm children with cerebral palsy. Neuroimage Clin 9:322–337
Chi L, Ke Y, Luo C, Gozal D, Liu R (2007) Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo. Neuroscience 144(3):991–1003
Chmatalova Z, Vyhnalek M, Laczo J, Hort J, Pospisilova R, Pechova M, Skoumalova A (2017) Relation of plasma selenium and lipid peroxidation end products in patients with Alzheimer’s disease. Physiol Res 66(6):1049–1056
Codazzi F, Hu A, Rai M, Donatello S, Salerno Scarzella F, Mangiameli E, Pelizzoni I, Grohovaz F, Pandolfo M (2016) Friedreich ataxia-induced pluripotent stem cell-derived neurons show a cellular phenotype that is corrected by a benzamide HDAC inhibitor. Hum Mol Genet 25(22):4847–4855
Connor JR, Menzies SL, Martin SMS, Mufson EJ (1992) A histochemical study of iron, transferrin, and ferritin in Alzheimer’s diseased brains. J Neurosci Res 31(1):75–83
Conrad M, Kagan VE, Bayir H, Pagnussat GC, Head B, Traber MG, Stockwell BR (2018) Regulation of lipid peroxidation and ferroptosis in diverse species. Genes Dev 32(9–10):602–619
Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, Andrews DF (1991) Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 337(8753):1304–1308
Davies P, Moualla D, Brown DR (2011) Alpha-synuclein is a cellular ferrireductase. PLoS One 6(1):e15814
Di Fonzo A, Ronchi D, Gallia F, Cribiu FM, Trezzi I, Vetro A, Della Mina E, Limongelli I, Bellazzi R, Ricca I, Micieli G, Fassone E, Rizzuti M, Bordoni A, Fortunato F, Salani S, Mora G, Corti S, Ceroni M, Bosari S, Zuffardi O, Bresolin N, Nobile-Orazio E, Comi GP (2014) Lower motor neuron disease with respiratory failure caused by a novel MAPT mutation. Neurology 82(22):1990–1998
Di Pietro V, Lazzarino G, Amorini AM, Tavazzi B, D’Urso S, Longo S, Vagnozzi R, Signoretti S, Clementi E, Giardina B, Lazzarino G, Belli A (2014) Neuroglobin expression and oxidant/antioxidant balance after graded traumatic brain injury in the rat. Free Radic Biol Med 69:258–264
Dietrich RB, Bradley WG Jr (1988) Iron accumulation in the basal ganglia following severe ischemic-anoxic insults in children. Radiology 168(1):203–206
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, Stockwell BR (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072
Do Van B, Gouel F, Jonneaux A, Timmerman K, Gele P, Petrault M, Bastide M, Laloux C, Moreau C, Bordet R, Devos D, Devedjian JC (2016) Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis 94:169–178
Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trumbach D, Mao G, Qu F, Bayir H, Fullekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M (2017) ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol 13(1):91–98
Duce JA, Tsatsanis A, Cater MA, James SA, Robb E, Wikhe K, Leong SL, Perez KA, Johanssen TJ, Greenough MA, Cho H-H, Galatis D, Moir RD, Masters CL, Mclean C, Tanzi RE, Cappai R, Barnham KJ, Ciccotosto GD, Rogers JT, Bush AI (2010) Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell 142(6):857–867
Duce JA, Wong BX, Durham H, Devedjian JC, Smith DP, Devos D (2017) Post translational changes to alpha-synuclein control iron and dopamine trafficking; a concept for neuron vulnerability in Parkinson’s disease. Mol Neurodegener 12(1):45
Feng G, Zhang Z, Bao Q, Zhang Z, Zhou L, Jiang J, Li S (2014) Protective effect of chinonin in MPTP-induced C57BL/6 mouse model of Parkinson’s disease. Biol Pharm Bull 37(8):1301–1307
Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M (2014) Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 16(12):1180–1191
Fu AL, Dong ZH, Sun MJ (2006) Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res 1109(1):201–206
Gao M, Monian P, Quadri N, Ramasamy R, Jiang X (2015) Glutaminolysis and transferrin regulate Ferroptosis. Mol Cell 59(2):298–308
Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, Heindel DW, Zuckerman DS, Bos PH, Reznik E, Ye LF, Tyurina YY, Lin AJ, Shchepinov MS, Chan AY, Peguero-Pereira E, Fomich MA, Daniels JD, Bekish AV, Shmanai VV, Kagan VE, Mahal LK, Woerpel KA, Stockwell BR (2018) FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol 14(5):507–515
Ghosh D, Levault KR, Brewer GJ (2014) Relative importance of redox buffers GSH and NAD (P) H in age-related neurodegeneration and Alzheimer disease-like mouse neurons. Aging Cell 13(4):631–640
Golko-Perez S, Amit T, Bar-Am O, Youdim MB, Weinreb O (2017) A novel iron chelator-radical scavenger ameliorates motor dysfunction and improves life span and mitochondrial biogenesis in SOD1(G93A) ALS mice. Neurotox Res 31(2):230–244
Hambright WS, Fonseca RS, Chen L, Na R, Ran Q (2017) Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol 12:8–17
Hanson LR, Roeytenberg A, Martinez PM, Coppes VG, Sweet DC, Rao RJ, Marti DL, Hoekman JD, Matthews RB, Frey WH 2nd, Panter SS (2009) Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther 330(3):679–686
Hider RC, Kong XL (2011) Glutathione: a key component of the cytoplasmic labile iron pool. Biometals 24(6):1179–1187
Ingold I, Aichler M, Yefremova E, Roveri A, Buday K, Doll S, Tasdemir A, Hoffard N, Wurst W, Walch A, Ursini F, Friedmann Angeli JP, Conrad M (2015) Expression of a catalytically inactive mutant form of glutathione peroxidase 4 (Gpx4) confers a dominant-negative effect in male fertility. J Biol Chem 290(23):14668–14678
Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T, Mehr L, Aichler M, Walch A, Lamp D, Jastroch M, Miyamoto S, Wurst W, Ursini F, Arner ESJ, Fradejas-Villar N, Schweizer U, Zischka H, Friedmann Angeli JP, Conrad M (2018) Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172(3):409–422 e421
Ito K, Eguchi Y, Imagawa Y, Akai S, Mochizuki H, Tsujimoto Y (2017) MPP+ induces necrostatin-1- and ferrostatin-1-sensitive necrotic death of neuronal SH-SY5Y cells. Cell Death Discov 3:17013
Izzet T, Osman K, Ethem U, Nihat Y, Ramazan K, Mustafa D, Hafize U, Riza KA, Birsen A, Habibe G, Seval A, Gonul S (2005) Oxidative stress in portal hypertension-induced rats with particular emphasis on nitric oxide and trace metals. World J Gastroenterol 11(23):3570–3573
Johnson WM, Wilson-Delfosse AL, Mieyal JJ (2012) Dysregulation of glutathione homeostasis in neurodegenerative diseases. Nutrients 4(10):1399–1440
Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H (2017) Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol 13(1):81–90
Kehrer JP (2000) The Haber-Weiss reaction and mechanisms of toxicity. Toxicology 149(1):43–50
Khalaf S, Ahmad AS, Chamara K, Dore S (2018) Unique properties associated with the brain penetrant iron chelator HBED reveal remarkable beneficial effects after brain trauma. J Neurotrauma
Klepac N, Relja M, Klepac R, Hecimovic S, Babic T, Trkulja V (2007) Oxidative stress parameters in plasma of Huntington’s disease patients, asymptomatic Huntington’s disease gene carriers and healthy subjects: a cross-sectional study. J Neurol 254(12):1676–1683
Klivenyi P, Andreassen OA, Ferrante RJ, Dedeoglu A, Mueller G, Lancelot E, Bogdanov M, Andersen JK, Jiang D, Beal MF (2000) Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci 20(1):1–7
Koeppen AH, Morral JA, McComb RD, Feustel PJ (2011) The neuropathology of late-onset Friedreich’s ataxia. Cerebellum 10(1):96–103
Kondo Y, Ogawa N, Asanuma M, Ota Z, Mori A (1995) Regional differences in late-onset iron deposition, ferritin, transferrin, astrocyte proliferation, and microglial activation after transient forebrain ischemia in rat brain. J Cereb Blood Flow Metab 15(2):216–226
Kuhn H, Banthiya S, van Leyen K (2015) Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta 1851(4):308–330
Kwan JY, Jeong SY, Van Gelderen P, Deng HX, Quezado MM, Danielian LE, Butman JA, Chen L, Bayat E, Russell J, Siddique T, Duyn JH, Rouault TA, Floeter MK (2012) Iron accumulation in deep cortical layers accounts for MRI signal abnormalities in ALS: correlating 7 tesla MRI and pathology. PLoS One 7(4):e35241
Lane DJR, Ayton S, Bush AI (2018) Iron and Alzheimer’s disease: an update on emerging mechanisms. J Alzheimers Dis 64(s1):S379–S395
Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BX, Adlard PA, Cherny RA, Lam LQ, Roberts BR, Volitakis I, Egan GF, McLean CA, Cappai R, Duce JA, Bush AI (2012) Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 18(2):291–295
Lei P, Ayton S, Appukuttan AT, Moon S, Duce JA, Volitakis I, Cherny R, Wood SJ, Greenough M, Berger G, Pantelis C, McGorry P, Yung A, Finkelstein DI, Bush AI (2017) Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Mol Psychiatry 22(3):396–406
Li X, Lei P, Tuo Q, Ayton S, Li QX, Moon S, Volitakis I, Liu R, Masters CL, Finkelstein DI, Bush AI (2015) Enduring elevations of hippocampal amyloid precursor protein and iron are features of beta-amyloid toxicity and are mediated by tau. Neurotherapeutics 12(4):862–873
Li Q, Han X, Lan X, Gao Y, Wan J, Durham F, Cheng T, Yang J, Wang Z, Jiang C, Ying M, Koehler RC, Stockwell BR, Wang J (2017) Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI Insight 2(7):e90777
Li QQ, Li Q, Jia JN, Liu ZQ, Zhou HH, Mao XY (2018) 12/15 lipoxygenase: a crucial enzyme in diverse types of cell death. Neurochem Int 118:34–41
Liu L, Huang W, Wang J, Song H, Cen J, Ji B (2017) Anthraquinone derivative exerted hormetic effect on the apoptosis in oxygen-glucose deprivation-induced PC12 cells via ERK and Akt activated Nrf2/HO-1 signaling pathway. Chem Biol Interact 262:1–11
Ma MW, Wang J, Zhang Q, Wang R, Dhandapani KM, Vadlamudi RK, Brann DW (2017) NADPH oxidase in brain injury and neurodegenerative disorders. Mol Neurodegener 12(1):7
Mathews CE, Leiter EH (1999) Constitutive differences in antioxidant defense status distinguish alloxan-resistant and alloxan-susceptible mice. Free Radic Biol Med 27(3–4):449–455
Mehdiratta M, Kumar S, Hackney D, Schlaug G, Selim M (2008) Association between serum ferritin level and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Stroke 39(4):1165–1170
Napoli E, Taroni F, Cortopassi GA (2006) Frataxin, iron-sulfur clusters, heme, ROS, and aging. Antioxid Redox Signal 8(3–4):506–516
National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24):1581–1587
Perez de la Ossa N, Sobrino T, Silva Y, Blanco M, Millan M, Gomis M, Agulla J, Araya P, Reverte S, Serena J, Davalos A (2010) Iron-related brain damage in patients with intracerebral hemorrhage. Stroke 41(4):810–813
Raven EP, Lu PH, Tishler TA, Heydari P, Bartzokis G (2013) Increased iron levels and decreased tissue integrity in hippocampus of Alzheimer’s disease detected in vivo with magnetic resonance imaging. J Alzheimers Dis 37(1):127–136
Shintoku R, Takigawa Y, Yamada K, Kubota C, Yoshimoto Y, Takeuchi T, Koshiishi I, Torii S (2017) Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci 108(11):2187–2194
Skouta R, Dixon SJ, Wang J, Dunn DE, Orman M, Shimada K, Rosenberg PA, Lo DC, Weinberg JM, Linkermann A, Stockwell BR (2014) Ferrostatins inhibit oxidative lipid damage and cell death in diverse disease models. J Am Chem Soc 136(12):4551–4556
Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascon S, Hatzios SK, Kagan VE, Noel K, Jiang X, Linkermann A, Murphy ME, Overholtzer M, Oyagi A, Pagnussat GC, Park J, Ran Q, Rosenfeld CS, Salnikow K, Tang D, Torti FM, Torti SV, Toyokuni S, Woerpel KA, Zhang DD (2017) Ferroptosis: a regulated cell death Nexus linking metabolism, redox biology, and disease. Cell 171(2):273–285
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, Liuyang ZY, Roisman L, Zhang ST, Ayton S, Wang Q, Crouch PJ, Ganio K, Wang XC, Pei L, Adlard PA, Lu YM, Cappai R, Wang JZ, Liu R, Bush AI (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11):1520–1530
Van Hoecke M, Prigent-Tessier A, Bertrand N, Prevotat L, Marie C, Beley A (2005) Apoptotic cell death progression after photothrombotic focal cerebral ischaemia: effects of the lipophilic iron chelator 2,2′-dipyridyl. Eur J Neurosci 22(5):1045–1056
Vinceti M, Chiari A, Eichmuller M, Rothman KJ, Filippini T, Malagoli C, Weuve J, Tondelli M, Zamboni G, Nichelli PF, Michalke B (2017) A selenium species in cerebrospinal fluid predicts conversion to Alzheimer’s dementia in persons with mild cognitive impairment. Alzheimers Res Ther 9(1):100
Welin AK, Svedin P, Lapatto R, Sultan B, Hagberg H, Gressens P, Kjellmer I, Mallard C (2007) Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr Res 61(2):153–158
Wenzel SE, Tyurina YY, Zhao J, Croix CMS, Dar HH, Mao G, Tyurin VA, Anthonymuthu TS, Kapralov AA, Amoscato AA, Mikulska-Ruminska K, Shrivastava IH, Kenny EM, Yang Q, Rosenbaum JC, Sparvero LJ, Emlet DR, Wen X, Minami Y, Qu F, Watkins SC, Holman TR, VanDemark AP, Kellum JA, Bahar I, Bayir H, Kagan VE (2017) PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell 171(3):628–641 e626
Wullner U, Loschmann PA, Schulz JB, Schmid A, Dringen R, Eblen F, Turski L, Klockgether T (1996) Glutathione depletion potentiates MPTP and MPP+ toxicity in nigral dopaminergic neurones. Neuroreport 7(4):921–923
Xu J, Wang H, Ding K, Zhang L, Wang C, Li T, Wei W, Lu X (2014) Luteolin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE pathway. Free Radic Biol Med 71:186–195
Yamamoto A, Shin R-W, Hasegawa K, Naiki H, Sato H, Yoshimasu F, Kitamoto T (2002) Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J Neurochem 82(5):1137–1147
Yang WS, Stockwell BR (2008) Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol 15(3):234–245
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA 113(34):E4966–E4975
Yigitkanli K, Pekcec A, Karatas H, Pallast S, Mandeville E, Joshi N, Smirnova N, Gazaryan I, Ratan RR, Witztum JL, Montaner J, Holman TR, Lo EH, van Leyen K (2013) Inhibition of 12/15-lipoxygenase as therapeutic strategy to treat stroke. Ann Neurol 73(1):129–135
Yoo MH, Gu X, Xu XM, Kim JY, Carlson BA, Patterson AD, Cai H, Gladyshev VN, Hatfield DL (2010) Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer’s disease. Antioxid Redox Signal 12(7):819–827
Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, Hoffmann PR, Ni JZ, Song GL (2017) Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and Autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci 37(9):2449–2462
Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, Guo SQ, Wang S, Guo T, Wang ZY, Guo C (2018) Alpha-lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol 14:535–548
Zhou ZD, Tan EK (2017) Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol Neurodegener 12(1):75
Zille M, Karuppagounder SS, Chen Y, Gough PJ, Bertin J, Finger J, Milner TA, Jonas EA, Ratan RR (2017) Neuronal death after hemorrhagic stroke in vitro and in vivo shares features of Ferroptosis and necroptosis. Stroke 48(4):1033–1043
Acknowledgements
The authors declare no conflict of interest. This work was supported by funds from the National Natural Science Foundation of China (81722016, 91632115, and 81571236).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, Jr., Tuo, Qz. & Lei, P. Ferroptosis, a Recent Defined Form of Critical Cell Death in Neurological Disorders. J Mol Neurosci 66, 197–206 (2018). https://doi.org/10.1007/s12031-018-1155-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-018-1155-6