Abstract
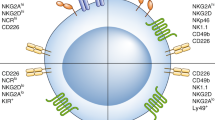
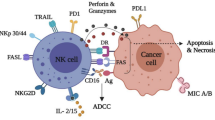
Natural killer (NK) cells are lymphoid cells of innate immunity that take important roles in immune surveillance. NK cells are considered as a bridge between innate and adaptive immunity, and their infiltration into tumor area is related positively with prolonged patient survival. They are defined as CD16+ CD56+ CD3− cells in clinic. NK cells promote cytolytic effects on target cells and induce their apoptosis. Loss of NK cell cytotoxic activity and reduction in the number of activating receptors are the current issues for application of such cells in cellular immunotherapy, which resulted in the diminished long-term effects. The focus of this review is to discuss about the activity of NK cells and cells with NK-like activity including natural killer T (NKT), cytokine-induced killer (CIK) and lymphokine-activated killer (LAK) cells in immunotherapy of human solid cancers.





Similar content being viewed by others
References
Narni-Mancinelli E, Vivier E, Kerdiles YM. The ‘T-cell-ness’ of NK cells: unexpected similarities between NK cells and T cells. Int Immunol. 2011;23(7):427–31.
Cózar B, et al. Tumor-infiltrating natural killer cells. Cancer Discov. 2021;11(1):34–44.
Abel AM, et al. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869.
Stabile H, et al. Role of distinct natural killer cell subsets in anticancer response. Front Immunol. 2017;8:293.
Nagai K, et al. Highly activated ex vivo-expanded natural killer cells in patients with solid tumors in a phase I/IIa clinical study. Anticancer Res. 2020;40(10):5687–700.
Kärre K. Natural killer cell recognition of missing self. Nat Immunol. 2008;9(5):477–80.
Mortezaee K. Myeloid-derived suppressor cells in cancer immunotherapy-clinical perspectives. Life Sci. 2021;277: 119627.
Yang Y, et al. Safety and short-term efficacy of irreversible electroporation and allogenic natural killer cell immunotherapy combination in the treatment of patients with unresectable primary liver cancer. Cardiovasc Intervent Radiol. 2019;42(1):48–59.
Lee HS, et al. Peripheral natural killer cell activity is associated with poor clinical outcomes in pancreatic ductal adenocarcinoma. J Gastroenterol Hepatol. 2020. https://doi.org/10.1111/jgh.15265.
Bähr I, et al. Obesity-associated alterations of natural killer cells and immunosurveillance of cancer. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.00245.
Kruse PH, et al. Natural cytotoxicity receptors and their ligands. Immunol Cell Biol. 2014;92(3):221–9.
Brillantes M, Beaulieu AM. Memory and memory-like NK cell responses to microbial pathogens. Front Cell Infect Microbiol. 2020;10:102.
Lonez C, et al. Study protocol for THINK: a multinational open-label phase I study to assess the safety and clinical activity of multiple administrations of NKR-2 in patients with different metastatic tumour types. BMJ Open. 2017. https://doi.org/10.1136/bmjopen-2017-017075.
Amin PJ, Shankar BS. Sulforaphane induces ROS mediated induction of NKG2D ligands in human cancer cell lines and enhances susceptibility to NK cell mediated lysis. Life Sci. 2015;126:19–27.
Fiuza-Luces C, et al. Effects of exercise on the immune function of pediatric patients with solid tumors: insights from the PAPEC randomized trial. Am J Phys Med Rehabil. 2017;96(11):831–7.
Bähr I, et al. Impaired natural killer cell subset phenotypes in human obesity. Immunol Res. 2018;66(2):234–44.
Izawa S, et al. H 2 O 2 production within tumor microenvironment inversely correlated with infiltration of CD56 dim NK cells in gastric and esophageal cancer: possible mechanisms of NK cell dysfunction. Cancer Immunol Immunother. 2011;60(12):1801–10.
Ishikawa T, et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int J Cancer. 2018;142(12):2599–609.
Pockley AG, Vaupel P, Multhoff G. NK cell-based therapeutics for lung cancer. Expert Opin Biol Ther. 2020;20(1):23–33.
Demaria O, et al. Natural killer cell engagers in cancer immunotherapy: next generation of immuno-oncology treatments. Eur J Immunol. 2021. https://doi.org/10.1002/eji.202048953.
Gauthier L, et al. Multifunctional natural killer cell engagers targeting NKp46 trigger protective tumor immunity. Cell. 2019;177(7):1701.e16-1713.e16.
Lim SH, et al. Effect of neoadjuvant chemoradiation on tumor-infiltrating/associated lymphocytes in locally advanced rectal cancers. Anticancer Res. 2014;34(11):6505–13.
Sage EK, et al. Effects of definitive and salvage radiotherapy on the distribution of lymphocyte subpopulations in prostate cancer patients. Strahlenther Onkol. 2017;193(8):648–55.
Liang S, et al. Tumor cryoablation in combination with natural killer cells therapy and Herceptin in patients with HER2-overexpressing recurrent breast cancer. Mol Immunol. 2017;92:45–53.
Tarhini AA, et al. NCI 8628—a randomized phase II study of Ziv-aflibercept and high dose interleukin-2 (HD IL-2) or HD IL-2 alone for inoperable stage III or IV melanoma. Cancer. 2018;124(22):4332.
Majidpoor J, Mortezaee K. Interleukin-2 therapy of cancer-clinical perspectives. Int Immunopharmacol. 2021;98:107836.
Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71(2):173–83.
Korbecki J, et al. CC chemokines in a tumor: a review of pro-cancer and anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci. 2020;21(20):7619.
Malchiodi ZX, Weiner LM. Understanding and targeting natural killer cell-cancer-associated fibroblast interactions in pancreatic ductal adenocarcinoma. Cancers. 2021;13(3):405.
Jorgovanovic D, et al. Roles of IFN-γ in tumor progression and regression: a review. Biomarker Research. 2020;8(1):1–16.
Böttcher JP, et al. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022.e14-1037.e14.
Mortezaee K, Majidpoor J. (Im) maturity in tumor ecosystem. Front Oncol. 2021;11:813897–813897.
Strauss J, et al. First-in-human phase I trial of a tumor-targeted cytokine (NHS-IL12) in subjects with metastatic solid tumors. Clin Cancer Res. 2019;25(1):99–109.
Margolin K, et al. Phase I trial of ALT-803, a novel recombinant IL15 complex, in patients with advanced solid tumors. Clin Cancer Res. 2018;24(22):5552–61.
Carrero RMS, et al. IL-15 is a component of the inflammatory milieu in the tumor microenvironment promoting antitumor responses. Proc Natl Acad Sci. 2019;116(2):599–608.
Souza-Fonseca-Guimaraes F, et al. Interferon-γ and granulocyte/monocyte colony-stimulating factor production by natural killer cells involves different signaling pathways and the adaptor stimulator of interferon genes (STING). J Biol Chem. 2013;288(15):10715–21.
Tarr PE. Granulocyte-macrophage colony-stimulating factor and the immune system. Med Oncol. 1996;13(3):133–40.
Nandagopal SA, et al. Dual roles of GM-CSF in modulating NK-cell migratory properties (CAM4P. 147). Am Assoc Immnol. 2015;35:585–99.
Hong I-S. Stimulatory versus suppressive effects of GM-CSF on tumor progression in multiple cancer types. Exp Mol Med. 2016;48(7):e242–e242.
Mortezaee K, Majidpoor J. Key promoters of tumor hallmarks. Int J Clin Oncol. 2021. https://doi.org/10.1007/s10147-021-02074-9.
Mortezaee K. Organ tropism in solid tumor metastasis: an updated review. Future Oncol. 2021;17(15):1943–61.
Najafi M, Mortezaee K, Majidpoor J. Stromal reprogramming: a target for tumor therapy. Life Sci. 2019;239: 117049.
Mortezaee K. Redox tolerance and metabolic reprogramming in solid tumors. Cell Biol Int. 2021;45(2):273–86.
Mortezaee K. Normalization in tumor ecosystem: opportunities and challenges. Cell Biol Int. 2021;45(10):2017–30.
Sarhan D, et al. Adaptive NK cells resist regulatory T-cell suppression driven by IL37. Cancer Immunol Res. 2018;6(7):766–75.
Abd Hamid M, et al. Enriched HLA-E and CD94/NKG2A interaction limits antitumor CD8+ tumor-infiltrating T lymphocyte responses. Cancer Immunol Res. 2019;7(8):1293–306.
Mortezaee K, Najafi M. Immune system in cancer radiotherapy: Resistance mechanisms and therapy perspectives. Crit Rev Oncol Hematol. 2020. https://doi.org/10.1016/j.critrevonc.2020.103180.
Farhood B, et al. Disruption of the redox balance with either oxidative or anti-oxidative overloading as a promising target for cancer therapy. J Cell Biochem. 2019;120(1):71–6.
Hagstrom AD, et al. The effect of resistance training on markers of immune function and inflammation in previously sedentary women recovering from breast cancer: a randomized controlled trial. Breast Cancer Res Treat. 2016;155(3):471–82.
Krijgsman D, Hokland M, Kuppen PJ. The role of natural killer T cells in cancer—a phenotypical and functional approach. Front Immunol. 2018;9:367.
Sebestyen Z, et al. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discovery. 2020;19(3):169–84.
Gütgemann S, et al. Cytokine-induced killer cells are type II natural killer T cells. GMS German Med Sci. 2007;5:7.
Wolf BJ, Choi JE, Exley MA. Novel approaches to exploiting invariant NKT cells in cancer immunotherapy. Front Immunol. 2018;9:384.
Shimizu K, et al. NK and NKT cell-mediated immune surveillance against hematological malignancies. Cancers. 2020;12(4):817.
Exley MA, et al. Adoptive transfer of invariant NKT cells as immunotherapy for advanced melanoma: a phase I clinical trial. Clin Cancer Res. 2017;23(14):3510–9.
Qin Y, et al. Invariant NKT cells facilitate cytotoxic T-cell activation via direct recognition of CD1d on T cells. Exp Mol Med. 2019;51(10):1–9.
Sangiolo D, et al. Alloreactivity and anti-tumor activity segregate within two distinct subsets of cytokine-induced killer (CIK) cells: implications for their infusion across major HLA barriers. Int Immunol. 2008;20(7):841–8.
Sangiolo D. Cytokine induced killer cells as promising immunotherapy for solid tumors. J Cancer. 2011;2:363.
Gao X, et al. Cytokine-induced killer cells as pharmacological tools for cancer immunotherapy. Front Immunol. 2017;8:774.
Lee JH, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148(7):1383.e6-1391.e6.
Olioso P, et al. Immunotherapy with cytokine induced killer cells in solid and hematopoietic tumours: a pilot clinical trial. Hematol Oncol. 2009;27(3):130–9.
Introna M. CIK as therapeutic agents against tumors. J Autoimmun. 2017;85:32–44.
Introna M, Correnti F. Innovative clinical perspectives for CIK cells in cancer patients. Int J Mol Sci. 2018;19(2):358.
Cui J, et al. Combined cellular immunotherapy and chemotherapy improves clinical outcome in patients with gastric carcinoma. Cytotherapy. 2015;17(7):979–88.
Kuhn C, Weiner HL. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy. 2016;8(8):889–906.
Chen D, et al. Cytokine-induced killer cells as a feasible adoptive immunotherapy for the treatment of lung cancer. Cell Death Dis. 2018;9(3):1–12.
Zhang Y, Schmidt-Wolf IG. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J Cell Physiol. 2020;235(12):9291–303.
Schmidt-Wolf I, et al. Phenotypic characterization and identification of effector cells involved in tumor cell recognition of cytokine-induced killer cells. Exp Hematol. 1993;21(13):1673–9.
Xia F, et al. Cytokine induced killer cells-assisted delivery of chlorin e6 mediated self-assembled gold nanoclusters to tumors for imaging and immuno-photodynamic therapy. Biomaterials. 2018;170:1–11.
Jiang J, Wu C, Lu B. Cytokine-induced killer cells promote antitumor immunity. J Transl Med. 2013;11(1):1–9.
Saito H, et al. A combined lymphokine-activated killer (LAK) cell immunotherapy and adenovirus-p53 gene therapy for head and neck squamous cell carcinoma. Anticancer Res. 2014;34(7):3365–70.
Pittari G, et al. Revving up natural killer cells and cytokine-induced killer cells against hematological malignancies. Front Immunol. 2015;6:230.
Yoshida Y, et al. Clinical study on the medical value of combination therapy involving adoptive immunotherapy and chemotherapy for stage IV colorectal cancer (COMVI Study). Anticancer Res. 2017;37(7):3941–6.
Sabry M, Lowdell MW. Killers at the crossroads: The use of innate immune cells in adoptive cellular therapy of cancer. Stem Cells Transl Med. 2020;9(9):974–84.
Li L, et al. Adoptive transfer of natural killer cells in combination with chemotherapy improves outcomes of patients with locally advanced colon carcinoma. Cytotherapy. 2018;20(1):134–48.
Lin M, et al. Prospective study of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced renal cell cancer. Immunol Lett. 2017;184:98–104.
Pérez-Martínez A, et al. A phase I/II trial of interleukin-15–stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy. 2015;17(11):1594–603.
Yang Y, et al. Phase I study of random healthy donor–derived allogeneic natural killer cell therapy in patients with malignant lymphoma or advanced solid tumors. Cancer Immunol Res. 2016;4(3):215–24.
Hoogstad-van Evert J, et al. Intraperitoneal infusion of ex vivo-cultured allogeneic NK cells in recurrent ovarian carcinoma patients (a phase I study). Medicine. 2019. https://doi.org/10.1097/MD.0000000000014290.
Gasser O, et al. A phase I vaccination study with dendritic cells loaded with NY-ESO-1 and α-galactosylceramide: induction of polyfunctional T cells in high-risk melanoma patients. Cancer Immunol Immunother. 2018;67(2):285–98.
Yu X, et al. A randomized phase II study of autologous cytokine-induced killer cells in treatment of hepatocelluar carcinoma. J Clin Immunol. 2014;34(2):194–203.
Lee J-H, et al. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. Cancer Immunol Immunother. 2019;68(1):23–32.
Chung MJ, et al. Phase II clinical trial of ex vivo-expanded cytokine-induced killer cells therapy in advanced pancreatic cancer. Cancer Immunol Immunother. 2014;63(9):939–46.
Savas B, Kerr PE, Pross HF. Lymphokine-activated killer cell susceptibility and adhesion molecule expression of multidrug resistant breast carcinoma. Cancer Cell Int. 2006;6(1):1–13.
Sakamoto N, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13(1):277.
Esser R, et al. NK cells engineered to express a GD2-specific antigen receptor display built-in ADCC-like activity against tumour cells of neuroectodermal origin. J Cell Mol Med. 2012;16(3):569–81.
Zhang C, et al. Cytokine-induced killer cells/natural killer cells combined with anti-GD2 monoclonal antibody increase cell death rate in neuroblastoma SK-N-SH cells. Oncol Lett. 2019;18(6):6525–35.
Federico SM, et al. A pilot trial of humanized anti-GD2 monoclonal antibody (hu14. 18K322A) with chemotherapy and natural killer cells in children with recurrent/refractory neuroblastoma. Clin Cancer Res. 2017;23(21):6441–9.
Xu Y-C, et al. Chemotherapy with or without autologous cytokine-induced killer cell transfusion as the first-line treatment for stage IV gastrointestinal cancer: a phase II clinical trial. J Cancer Res Clin Oncol. 2016;142(6):1315–23.
Zhao H, et al. Autologous cytokine-induced killer cells improves overall survival of metastatic colorectal cancer patients: results from a phase II clinical trial. Clin Colorectal Cancer. 2016;15(3):228–35.
Kong D-S, et al. Phase III randomized trial of autologous cytokine-induced killer cell immunotherapy for newly diagnosed glioblastoma in Korea. Oncotarget. 2017;8(4):7003.
Li N, et al. Combined treatment with autologous CIK cells, radiotherapy and chemotherapy in advanced cervical cancer. Pathol Oncol Res. 2019;25(2):691–6.
Li X, et al. Phase II/III study of radiofrequency ablation combined with cytokine-induced killer cells treating colorectal liver metastases. Cell Physiol Biochem. 2016;40(1–2):137–45.
Wang S, et al. DC-CIK as a widely applicable cancer immunotherapy. Expert Opin Biol Ther. 2020;20(6):601–7.
Märten A, et al. Interactions between dendritic cells and cytokine-induced killer cells lead to an activation of both populations. J Immunother. 2001;24(6):502–10.
Zhang Y, et al. Clinical studies applying cytokine-induced killer cells for the treatment of renal cell carcinoma. Cancers. 2020;12(9):2471.
Yang T, et al. Co-culture of dendritic cells and cytokine-induced killer cells effectively suppresses liver cancer stem cell growth by inhibiting pathways in the immune system. BMC Cancer. 2018;18(1):1–10.
Du H, Yang J, Zhang Y. Cytokine-induced killer cell/dendritic cell combined with cytokine-induced killer cell immunotherapy for treating advanced gastrointestinal cancer. BMC Cancer. 2020;20:1–11.
Mu Y, et al. Effectiveness and safety of chemotherapy combined with cytokine-induced killer cell/dendritic cell–cytokine-induced killer cell therapy for treatment of gastric cancer in China: a systematic review and meta-analysis. Cytotherapy. 2016;18(9):1162–77.
Hu J, et al. Effect and safety of cytokine-induced killer (CIK) cell immunotherapy in patients with breast cancer: a meta-analysis. Medicine. 2017. https://doi.org/10.1097/MD.0000000000008310).
Wang D, et al. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC Cancer. 2014;14(1):1–7.
Zhao X, et al. Cytokine induced killer cell-based immunotherapies in patients with different stages of renal cell carcinoma. Cancer Lett. 2015;362(2):192–8.
Jiang N, et al. Dendritic cell/cytokine-induced killer cell immunotherapy combined with S-1 in patients with advanced pancreatic cancer: a prospective study. Clin Cancer Res. 2017;23(17):5066–73.
Wang M, et al. S-1 plus CIK as second-line treatment for advanced pancreatic cancer. Med Oncol. 2013;30(4):747.
Zhao P, et al. Dendritic cell immunotherapy combined with cytokine-induced killer cells promotes skewing toward Th2 cytokine profile in patients with metastatic non-small cell lung cancer. Int Immunopharmacol. 2015;25(2):450–6.
Zhao Y, et al. Combination of DC/CIK adoptive T cell immunotherapy with chemotherapy in advanced non-small-cell lung cancer (NSCLC) patients: a prospective patients’ preference-based study (PPPS). Clin Transl Oncol. 2019;21(6):721–8.
Song H, et al. Increased cycles of DC/CIK immunotherapy decreases frequency of Tregs in patients with resected NSCLC. Int Immunopharmacol. 2017;52:197–202.
Szlasa W, et al. Oxidative effects during irreversible electroporation of melanoma cells—in vitro study. Molecules. 2021;26(1):154.
Lin M, et al. Percutaneous irreversible electroporation combined with allogeneic natural killer cell immunotherapy for patients with unresectable (stage III/IV) pancreatic cancer: a promising treatment. J Cancer Res Clin Oncol. 2017;143(12):2607–18.
Lin M, et al. Clinical efficacy of percutaneous cryoablation combined with allogenic NK cell immunotherapy for advanced non-small cell lung cancer. Immunol Res. 2017;65(4):880–7.
Mortezaee, K., Immune escape: A critical hallmark in solid tumors. Life Sciences, 2020: 118110.
Kirkin AF, et al. Adoptive cancer immunotherapy using DNA-demethylated T helper cells as antigen-presenting cells. Nat Commun. 2018;9(1):1–12.
Rezvani K. Adoptive cell therapy using engineered natural killer cells. Bone Marrow Transplant. 2019;54(2):785–8.
Liu E, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545–53.
Müller, N., et al., Engineering NK cells modified with an EGFRvIII-specific chimeric antigen receptor to overexpress CXCR4 improves immunotherapy of CXCL12/SDF-1α-secreting glioblastoma. Journal of immunotherapy (Hagerstown, Md.: 1997), 2015. 38(5): 197.
Heczey A, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. 2020;26(11):1686–90.
Zhou Y, et al. Effects of preemptive analgesia with flurbiprofen ester on lymphocytes and natural killer cells in patients undergoing esophagectomy: a randomized controlled pilot study. Thoracic cancer. 2017;8(6):649–54.
Shi L, et al. Laparoscopic surgery versus open surgery for colorectal cancer: impacts on natural killer cells. Cancer Control. 2020;27(1):1073274820906811.
Oh C-S, et al. Effect of equipotent doses of propofol versus sevoflurane anesthesia on regulatory T cells after breast cancer surgery. Anesthesiology. 2018;129(5):921–31.
Desmond F, et al. Effect of anaesthetic technique on immune cell infiltration in breast cancer: a follow-up pilot analysis of a prospective, randomised, investigator-masked study. Anticancer Res. 2015;35(3):1311–9.
Cho JS, et al. The effects of perioperative anesthesia and analgesia on immune function in patients undergoing breast cancer resection: a prospective randomized study. Int J Med Sci. 2017;14(10):970.
Mortezaee K. Enriched cancer stem cells, dense stroma, and cold immunity: Interrelated events in pancreatic cancer. J Biochem Mol Toxicol. 2021. https://doi.org/10.1186/s12885-018-4064-8.
Lim J-A, et al. The effect of propofol and sevoflurane on cancer cell, natural killer cell, and cytotoxic T lymphocyte function in patients undergoing breast cancer surgery: an in vitro analysis. BMC Cancer. 2018;18(1):159.
Smyth MJ, et al. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2(11):850–61.
Gallois A, et al. Reversal of natural killer cell exhaustion by TIM-3 blockade. Oncoimmunology. 2014;3(12): e946365.
Zheng Y, et al. TNF-α-induced Tim-3 expression marks the dysfunction of infiltrating natural killer cells in human esophageal cancer. J Transl Med. 2019;17(1):1–12.
Silva IG, et al. The Tim-3-galectin-9 secretory pathway is involved in the immune escape of human acute myeloid leukemia cells. EBioMedicine. 2017;22:44–57.
Majidpoor, J. and K. Mortezaee, Interleukin-6 in SARS-CoV-2 induced disease: Interactions and therapeutic applications. Biomedicine & Pharmacotherapy, 2021: 112419.
Mortezaee K. Hypoxia induces core-to-edge transition of progressive tumoral cells: A critical review on differential yet corroborative roles for HIF-1α and HIF-2α. Life Sci. 2020;242:117145.
Mortezaee K, Majidpoor J. The impact of hypoxia on immune state in cancer. Life Sci. 2021;286: 120057.
Mortezaee K, et al. Metformin as a radiation modifier; implications to normal tissue protection and tumor sensitization. Curr Clin Pharmacol. 2019;14(1):41–53.
Yuen VW-H, Wong CC-L. Hypoxia-inducible factors and innate immunity in liver cancer. J Clin Investig. 2020;130(10):5052–62.
You L, et al. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med Res Rev. 2020. https://doi.org/10.1002/med.21771.
Mortezaee K, et al. Post-treatment of melatonin with CCl4 better reduces fibrogenic and oxidative changes in liver than melatonin co-treatment. J Cell Biochem. 2018;119(2):1716–25.
Mortezaee K. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and liver fibrosis: a review. Cell Biochem Funct. 2018;36(6):292–302.
Mortezaee K, et al. Resveratrol as an adjuvant for normal tissues protection and tumor sensitization. Curr Cancer Drug Targets. 2020;20(2):130–45.
Foulds GA, et al. Immune-phenotyping and transcriptomic profiling of peripheral blood mononuclear cells from patients with breast cancer: identification of a 3 gene signature which predicts relapse of triple negative breast cancer. Front Immunol. 2018;9:2028.
Temam S, et al. An exploratory, open-label, randomized, multicenter study to investigate the pharmacodynamics of a glycoengineered antibody (imgatuzumab) and cetuximab in patients with operable head and neck squamous cell carcinoma. Ann Oncol. 2017;28(11):2827–35.
Fossati M, et al. Immunological changes in the ascites of cancer patients after intraperitoneal administration of the bispecific antibody catumaxomab (anti-EpCAM× anti-CD3). Gynecol Oncol. 2015;138(2):343–51.
Jahn J, et al. Decreased NK cell functions in obesity can be reactivated by fat mass reduction. Obesity. 2015;23(11):2233–41.
Mortezaee K, Majidpoor J. Checkpoint inhibitor/interleukin-based combination therapy of cancer. Cancer Med. 2022. https://doi.org/10.1002/cam4.4659.
O’Shea D, Hogan AE. Dysregulation of natural killer cells in obesity. Cancers. 2019;11(4):573.
Majidpoor J, Mortezaee K. Steps in metastasis: an updated review. Med Oncol. 2021;38(1):1–17.
Messaoudene M, et al. Characterization of the microenvironment in positive and negative sentinel lymph nodes from melanoma patients. PLoS ONE. 2015;10(7):e0133363.
Nakamura K, Smyth MJ. Immunoediting of cancer metastasis by NK cells. Nature Cancer. 2020;1(7):670–1.
Wu S-Y, et al. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):1–26.
Muraro E, et al. Improved natural killer cell activity and retained anti-tumor CD8+ T cell responses contribute to the induction of a pathological complete response in HER2-positive breast cancer patients undergoing neoadjuvant chemotherapy. J Transl Med. 2015;13(1):1–14.
Wang F, et al. TIGIT expression levels on human NK cells correlate with functional heterogeneity among healthy individuals. Eur J Immunol. 2015;45(10):2886–97.
Najafi M, et al. The current knowledge concerning solid cancer and therapy. J Biochem Mol Toxicol. 2021;35(11):e22900.
Chan E, et al. Open-label phase 1b study of FOLFIRI plus cetuximab plus IMO-2055 in patients with colorectal cancer who have progressed following chemotherapy for advanced or metastatic disease. Cancer Chemother Pharmacol. 2015;75(4):701–9.
van den Hout MF, et al. Local delivery of CpG-B and GM-CSF induces concerted activation of effector and regulatory T cells in the human melanoma sentinel lymph node. Cancer Immunol Immunother. 2016;65(4):405–15.
Samanta D, et al. BIRC2 expression impairs anti-cancer immunity and immunotherapy efficacy. Cell Rep. 2020;32(8): 108073.
Mortezaee K, et al. Melatonin pretreatment enhances the homing of bone marrow-derived mesenchymal stem cells following transplantation in a rat model of liver fibrosis. Iran Biomed J. 2016;20(4):207.
Ding Q, et al. CXCL9: evidence and contradictions for its role in tumor progression. Cancer Med. 2016;5(11):3246–59.
Acknowledgements
This work received ethical approval (Ethical code: IR.MUK.REC.1400.013) from Kurdistan University of Medical Sciences.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
K.M gave the conceptualization. K.M and J.M wrote the initial manuscript. Final revisions were made by K.M. Articles was selected by K.M. Both authors approved the final draft.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mortezaee, K., Majidpoor, J. NK and cells with NK-like activities in cancer immunotherapy-clinical perspectives. Med Oncol 39, 131 (2022). https://doi.org/10.1007/s12032-022-01735-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-022-01735-7