Abstract
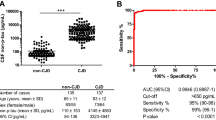
At present, the testing of 14-3-3 protein in cerebrospinal fluid (CSF) is a standard biomarker test in suspected sporadic Creutzfeldt-Jakob disease (sCJD) diagnosis. Increasing 14-3-3 test referrals in CJD reference laboratories in the last years have led to an urgent need to improve established 14-3-3 test methods. The main result of our study was the validation of a commercially available 14-3-3 ELISA next to the commonly used Western blot method as a high-throughput screening test. Hereby, 14-3-3 protein expression was quantitatively analyzed in CSF of 231 sCJD and 2035 control patients. We obtained excellent sensitivity/specificity values of 88 and 96 % that are comparable to the established Western blot method. Since standard protocols and preanalytical sample handling have become more important in routine diagnostic, we investigated in a further step the reproducibility and stability of 14-3-3 as a biomarker for human prion diseases. Ring trial data from 2009 to 2013 revealed an increase of Fleiss’ kappa from 0.51 to 0.68 indicating an improving reliability of 14-3-3 protein detection. The stability of 14-3-3 protein under short-term and long-term storage conditions at various temperatures and after repeated freezing/thawing cycles was confirmed. Contamination of CSF samples with blood appears likely to be an important factor at a concentration of more than 2500 erythrocytes/μL. Hemolysis of erythrocytes with significant release of 14-3-3 protein started after 2 days at room temperature. We first define clear standards for the sample handling, short- and long-term storage of CSF samples as well as the handling of blood- contaminated samples which may result in artificially elevated CSF levels of 14-3-3.





Similar content being viewed by others
References
Broadie K, Rushton E, Skoulakis E, Davis R (1997) Leonardo, a Drosophila 14-3-3 protein involved in learning, regulates presynaptic function. Neuron 2:391–402
van Hemert M, Steensma H, van Heusden G (2001) 14-3-3 proteins: key regulators of cell division, signalling and apoptosis. Bioessays 10:936–946
Zerr I, Bodemer M, Gefeller O, Otto M, Poser S, Wiltfang J, Windl O, Kretzschmar HA, Weber T (1998) Detection of 14-3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt-Jakob disease. Ann Neurol 43:32–40
Zerr I, Bodemer M, Weber T (1997) The 14-3-3 brain protein and transmissible spongiform encephalopathy [letter]. N Engl J Med 336:874
Zerr I, Pocchiari M, Collins S, Brandel JP, de Pedro CJ, Knight RSG, Bernheimer H, Cardone F, Delasnerie-Lauprêtre N, Cuadrado Corrales N, Ladogana A, Fletcher A, Bodemer M, Awan T, Ruiz Bremón A, Budka H, Laplanche JL, Will RG, Poser S (2000) Analysis of EEG and CSF 14-3-3 proteins as aids to the diagnosis of Creutzfeldt-Jakob disease. Neurology 55:811–815
Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, Ladogana A, Schuur M, Haik S, Collins SJ, Jansen GH, Stokin GB, Pimentel J, Hewer E, Collie DA, Smith P, Varges D, Heinemann U, Meissner B, Roberts H, Brandel JP, Van Dujin CM, Pocchiari M, Begue P, Cras P, Will RG, Sanchez-Juan P (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132:2659–2668
Gmitterová K, Heinemann U, Bodemer M, Krasnianski A, Meissner B, Kretzschmar HA, Zerr I (2008) 14-3-3 CSF levels in sporadic Creutzfeldt-Jakob disease differ across molecular subtypes. Neurobiol Aging 30:842–850
WHO (1998) Human transmissible spongiform encephalopathies. Wkly Epidemiol Rec 47:361–365
Schmitz M, Lüllmann K, Zafar S, Ebert E, Wohlhage M, Oikonomou P, Schlomm M, Mitrova E, Beekes M, Zerr I (2014) Association of prion protein genotype and scrapie prion protein type with cellular prion protein charge isoform profiles in cerebrospinal fluid of humans with sporadic of familial prion diseases. Neurobiol Aging 35:1177–1188
Schmitz M, Schlomm M, Hasan B, Beekes M, Mitrova E, Korth C, Brell A, Carimalo J, Gawinecka J, Varges D, Zerr I (2010) Codon 129 polymorphism and the E200K mutation do not affect the cellular prion protein isoform composition in the cerebrospinal fluid from patients with Creutzfeldt-Jakob disease. Eur J Neurosci 31:2024–2031
Puoti G, Bizzi A, Forloni G, Safar JG, Tagliavini F, Gambetti P (2012) Sporadic human prion diseases: molecular insights and diagnosis. Lancet Neurol 11:618–628
Stoeck K, Sanchez-Juan P, Gawinecka J, Green A, Ladogana A, Pocchiari M, Sanchez-Valle R, Mitrova E, Sklaviadis T, Kulczycki J, Slivarichova D, Saiz A, Calero M, Knight R, Aguzzi A, Laplanche JL, Peoc’h K, Schelzke G, Karch A, van Duijn CM, Zerr I (2012) Cerebrospinal fluid biomarker supported diagnosis of Creutzfeldt-Jakob disease and rapid dementias: a longitudinal multicentre study over 10 years. Brain 135:3051–3061
Beaudry P, Cohen P, Brandel JP, Delasnerie-Laupretre N, Richard S, Launay JM, Laplanche JL (1999) 14-3-3 protein, neuron-specific enolase, and S-100 protein in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Dement Geriatr Cogn Disord 10:40–46
Geschwind M, Martindale J, Miller D, De Armond SJ, Uyehara-Lock J, Gaskin D, Kramer JH, Barbaro NM, Miller BL (2003) Challenging the clinical utility of the 14-3-3 protein for the diagnosis of sporadic Creutzfeldt-Jakob disease. Arch Neurol 60:813–816
Cuadrado-Corrales N, Jiménez-Huete A, Albo C, Hortiguela R, Vega L, Cerrato L, Sierra-Moros M, Rábano A, de Pedro-Cuesta J, Calero M (2006) Impact of the clinical context on the 14-3-3 test for the diagnosis of sporadic CJD. BMC Neurol 6:25
Green AJ (2002) Use of 14-3-3 in the diagnosis of Creutzfeldt-Jakob disease. Biochem Soc Symp 30:382–386
Green AJ, Thompson EJ, Stewart GE, Zeidler M, McKenzie JM, MacLeod M-A, Ironside JW, Will RG, Knight RS (2001) Use of 14-3-3 and other brain-specific proteins in CSF in the diagnosis of variant Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry 70:744–748
Zerr I, Bodemer M, Räcker S, Grosche S, Poser S, Kretzschmar HA, Weber T (1995) Cerebrospinal fluid concentration of neuron-specific enolase in diagnosis of Creutzfeldt-Jakob disease. Lancet 345:1609–1610
Zerr I, Bodemer M, Westermann R, Schröter A, Jacobi C, Arlt S, Otto M, Poser S (2000) 14-3-3 proteins in neurological disorders. J Neurol 247(Suppl 3):III/14
Matsui Y, Satoh K, Miyazaki T, Shirabe S, Atarashi R, Mutsukura K, Satoh A, Kataoka Y, Nishida N (2011) High sensitivity of an ELISA kit for dedection of the gamma-isoform of 14-3-3 proteins: usefulness in laboratory diagnosis of human prion disease. BMC Neurol 11, doi:10.1186/471-2377-11-120
Atarashi R, Sano K,Fuse T, Yamaguchi N, Ishibashi D, Matsubara T, Nakagaki T, Yamanaka H, Shirabe S, Yamada M, Mizusawa H, Kitamoto T, Klug G, McGlade A, Collins SJ, Nishida N (2011) Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion.Nat Med 17:175–178
Cramm M, Schmitz M, Karch A, Zafar S, Varges D, Mitrova E, Schroeder B, Raeber AJ, Kuhn F, Zerr I (2015) Characteristic CSF prion-seeding efficiency in humans with prion diseases. Mol Neurobiol 51:396–405
Cramm M, Schmitz M, Zafar S, Karch A, Mitrova E, Schroeder B, Raeber A, Kuhn F, Satoh K, Collins S, Zerr I (2015) Stability and reproducibility underscore utility of RT-QuIC CSF analysis for diagnosis of human prion disease, in press
McGuire LI, Peden AH, Orrú CD, Wilham JM, Appleford NE, Mallinson G, Andrews M, Head MW, Caughey B, Will RG, Knight RS, Green AJ (2012) Real time quaking-induced conversion analysis of cerebrospinal fluid in sporadic Creutzfeldt-Jakob disease. Ann Neurol 72:278–285
Sano K, Satoh K, Atarashi R, Takashima H, Iwasaki Y, Yoshida M, Sanjo N, Murai H, Mizusawa H, Schmitz M, Zerr I, Kim YS, Nishida N (2013) Early detection of abnormal prion protein in genetic human prion diseases now possible using real-time QUIC assay. PLoS ONE 8:e54915
Coulthart MB, Jansen GH, Olsen E, Godal DL, Connolly T, Choi BC, Wang Z, Cashman NR (2011) Diagnostic accuracy of cerebrospinal fluid protein markers for sporadic Creutzfeldt-Jakob disease in Canada: a 6-year prospective study. BMC Neurol 11:133
Sanchez-Juan P, Green A, Ladogana A, Cuadrado-Corrales N, Sanchez-Valle R, Mitrova E, Stoeck K, Sklaviadis T, Kulczycki J, Hess K, Bodemer M, Slivarichova D, Saiz A, Calero M, Ingrosso L, Knight R, Janssens C, Van Duijn C, Zerr I (2006) CSF tests in the differential diagnosis of Creutzfeldt-Jakob disease. Neurology 67:637–643
Ramont L, Thoannes H, Volondat A, Chastang F, Millet MC, Maquart FX (2005) Effects of hemolysis and storage condition on neuron-specific enolase (NSE) in cerebrospinal fluid and serum: implications in clinical practice. Clin Chem Lab Med 43:1215–1217
Jensen M, Hartmann T, Engvall B, Wang R, Uljon SN, Sennvik K, Näslund J, Muehlhauser F, Nordstedt C, Beyreuther K, Lannfelt L (2000) Quantification of Alzheimer amyloid beta peptides ending at residues 40 and 42 by novel ELISA systems. Mol Med 6:291–302
Schoonenboom NS, Mulder C, Vanderstichele H, Van Elk EJ, Kok A, Van Kamp GJ, Scheltens P, Blankenstein MA (2005) Effects of processing and storage conditions on amyloid beta (1–42) and tau concentrations in cerebrospinal fluid: implications for use in clinical practice. Clin Chem 51:189–195
Vanderstichele H, Van Kerschaver E, Hesse C, Davidsson P, Buyse MA, Andreasen N, Minthon L, Wallin A, Blennow K, Vanmechelen E (2000) Standardization of measurement of beta-amyloid (1–42) in cerebrospinal fluid and plasma. Amyloid 7:245–258
Vandermeeren M, Mercken M, Vanmechelen E, Six J, van de Voorde A, Martin JJ, Cras P (1993) Detection of tau proteins in normal and Alzheimer’s disease cerebrospinal fluid with a sensitisve sandwich enzyme-linked immunosorbent assay. J Neurochem 61:1828–1834
Hsich G, Kenney K, Gibbs CJ Jr, Lee KH, Harrington MG (1996) The 14-3-3 brain protein in cerebrospinal fluid as a marker for transmissible spongifrom encephalopathies. N Engl J Med 335:924–930
Lemstra AW, van Meegen MT, Vreyling JP, Meijerink PH, Jansen GH, Bulk S, Baas F, van Gool WA (2000) 14-3-3 testing in diagnosing Creutzfeldt-Jakob disease: a prospective study in 112 patients. Neurology 55:514–516
Kenney K, Brechtel C, Takahashi H, Kurohara K, Anderson P, Gibbs CJ Jr (2000) An enzyme-linked immunosorbent assay to quantify 14-3-3 proteins in the cerebrospinal fluid of suspected Creutzfeldt-Jakob disease patients. Ann Neurol 48:395–398
Collins S, Boyd A, Fletcher A, Gonzales M, McLean CA, Byron K, Masters CL (2000) Creutzfeldt-Jakob disease: diagnostic utility of 14-3-3 protein immunodetection in cerebrospinal fluid. J Clin Neurosci 7:203–208
Van Everbroeck B, Quoilin S, Boons J, Martin JJ, Cras P (2003) A prospective study of CSF markers in 250 patients with possible Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry 74:1210–1214
Castellani RJ, Colucci M, Xie Z, Zou W, Li C, Parchi P, Capellari S, Pastore M, Rahbar MH, Chen SG, Gambetti P (2004) Sensitivity of 14-3-3 protein test varies in subtypes of sporadic Creutzfeldt-Jakob disease. Neurology 63:436–442
Collins SJ, Sanchez-Juan P, Masters CL, Klug GM, van Duijn C, Poleggi A, Pocchiari M, Almonti S, Cuadrado-Corrales N, de Pedro-Cuesta J, Budka H, Gelpi E, Glatzel M, Tolnay M, Hewer E, Zerr I, Heinemann U, Kretzschmar HA, Jansen GH, Olsen E, Mitrova E, Alpérovitsch A, Brandel JP, Mackenzie J, Murray K, Will RG (2006) Determinants of diagnostic investigation sensitivities across the clinical spectrum of sporadic Creutzfeldt-Jakob disease. Brain 129:2278–2287
Baldeiras IE, Ribeiro MH, Pacheco P, Machado A, Santana I, Cunha L, Oliveira C (2009) Diagnostic value of CSF protein profile in a Portuguese population of sCJD patients. J Neurol 256:1540–1550
Begué P, Martinetto H, Schultz M, Rojas E, Romero C, D’Giano C, Sevlever G, Somoza M, Taratuto A (2011) Creutzfeldt-Jakob disease surveillance in Argentina, 1997–2008. Neuroepidemiology 37:193–202
Hamlin C, Puoti G, Berri S, Sting E, Harris C, Cohen M, Spear C, Bizzi A, Debanne SM, Rowland DY (2012) A comparison of tau and 14-3-3 protein in the diagnosis of Creutzfeldt-Jakob disease. Neurology 79:547–552
Chohan G, Pennington C, Mackenzie J, Andrews M, Everington D, Will R, Knight R, Green A (2010) The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt-Jakob disease in the UK: a 10-year review. J Neurol Neurosurg Psychiatry 81:1243–1248
Acknowledgments
The study was performed within the recently established Clinical Dementia Center at the University Medical Center Göttingen and was supported by grants from the EU Joint Program–Neurodegenerative Disease Research [JPND-DEMTEST (Biomarker based diagnosis of rapid progressive dementias-optimization of diagnostic protocols, 01ED1201A)]. This study was also partly supported by the Robert Koch Institute through funds from the Federal Ministry of Health (grant no. 1369–341) and by a grant from the European Commission (Protecting the food chain from prions: shaping European priorities through basic and applied research (PRIORITY, No. 222887) Project number: FP7-KBBE-2007-2A). Thanks to Michele Equestre for technical assistance. The Australian National CJD Registry is funded by the Commonwealth Department of Health and S Collins is supported by a NHMRC Practitioner Fellowship (#APP1005816).
Conflict of Interests
On behalf of all authors, the corresponding author states that there are no conflicts of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplement 1
Determination of 14-3-3 protein level in CSF from neurodegenerative and non-neurodegenerative diseases. No significant differences in 14-3-3 protein level could be observed. (GIF 13 kb)
High Resolution Image
(TIFF 3982 kb)
Supplement 2
Comparison of diagnostic accuracy of 14-3-3 protein to tau and S100B protein. The diagnostic accuracy of 14-3-3 protein was analysed in comparison to tau and S100B which showed the lowest accuracy. The combination of different biomarker proteins increased the diagnostic accuracy further. (GIF 121 kb)
High Resolution Image
(TIFF 7263 kb)
Rights and permissions
About this article
Cite this article
Schmitz, M., Ebert, E., Stoeck, K. et al. Validation of 14-3-3 Protein as a Marker in Sporadic Creutzfeldt-Jakob Disease Diagnostic. Mol Neurobiol 53, 2189–2199 (2016). https://doi.org/10.1007/s12035-015-9167-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9167-5