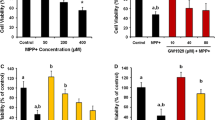
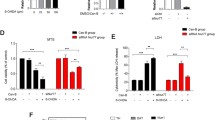
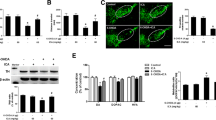
Abstract
Recent evidence suggests that nerve growth factor IB (Nur77) and nuclear receptor related1 (Nurr1) are differentially involved in dopaminergic neurodegeneration. Since memantine has shown clinically relevant efficacy in Parkinson’s disease (PD) and displayed a potent protective effect on dopaminergic neurons in experimental PD models, we asked if it exerts its neuroprotection by regulating Nur77 and Nurr1 signaling. We adopted a well-established in vitro PD model, 6-hydroxydopamine (OHDA)-lesioned PC12 cells, to test our hypothesis. Different concentrations of memantine were incubated with 6-OHDA-lesioned PC12 cells, and Nur77/Nurr1 and their related signaling molecules were examined by Western blot and immunocytochemistry. Nur77-deficient PC12 cells were used to verify the influences of Nur77 on neurodegeneration and memantine-mediated neuroprotection. We found that memantine reversed Nur77 upregulation and restored Nurr1 downregulation in 6-OHDA-lesioned PC12 cells. 6-OHDA incubation caused Nur77 translocation from the nucleus to cytosol and induced co-localization of Cyt c/HSP60/Nur77 in the cytosol. Memantine strongly reduced the sub-cellular translocations of Nur77/Cyt c/HSP60 under 6-OHDA-induced oxidative condition. Knockdown of Nur77 enhanced the viability of PC12 cells exposed to 6-OHDA, while memantine-induced neuroprotection was much less in the cells with Nur77 knockdown than in those without it. We conclude that Nur77 plays a crucial role in modulating mitochondrial impairment and contributes to neurodegeneration under the experimental PD condition. Memantine effectively suppresses such Nur77-mediated neurodegeneration and promotes survival signaling through post-translational modification of Nurr1. Nur77 and Nurr1 present a contra-directionally coupling interaction in memantine-mediated neuroprotection.







Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- AKT:
-
Also known as protein kinase B
- Annexin V-FITC:
-
Annexin V-fluorescein isothiocyanate
- ANOVA:
-
Analysis of variance
- BCA:
-
Bicinchoninic acid
- BDNF:
-
Brain-derived neurotrophic factor
- BSA:
-
Bovine serum albumin
- CCK8:
-
Cell Counting Kit-8
- Cyt c:
-
Cytochrome c
- DA:
-
Dopamine
- DAT:
-
Dopamine transporter
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DNA:
-
Deoxyribonucleic acid
- EDTA:
-
Ethylene diamine tetraacetic acid
- EGTA:
-
Ethylene glycol tetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- ERK:
-
Extracellular regulated protein kinases
- FBS:
-
Fetal bovine serum
- HDAC:
-
Histone deacetylase
- HEPES:
-
N-2-hydroxyethyl piperazine-N0-2-ethanesulfonic acid
- HSP60:
-
Heat shock protein 60
- IL-1:
-
Interleukin-1
- IL-6:
-
Interleukin-6
- JNK:
-
c-Jun N-terminal kinase
- KCL:
-
Potassium chloride
- LBD:
-
Dementia with Lewy bodies
- LDH:
-
Lactate dehydrogenase
- MAPK:
-
Mitogen-activated protein kinases
- NaF:
-
Sodium fluoride
- NaCl:
-
Sodium chloride
- NMDA:
-
N-Methyl-d-aspartate
- NMDAR:
-
N-Methyl-d-aspartate receptor
- NR4A1:
-
Nuclear receptor subfamily 4, group A, member 1
- Nur77:
- Nurr1:
- OD:
-
Optical density
- PBS:
-
Phosphate buffered saline
- PC12 cells:
-
Pheochromocytoma 12 cells
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson’s disease dementia
- PI:
-
Propidium iodide
- PI3K:
-
Phosphatidylinositol 3 kinase
- PVDF:
-
Polyvinylidene fluoride
- RNA:
-
Ribonucleic acid
- RPMI:
-
Roswell Park Memorial Institute
- RQ-PCR:
-
Real-time quantitative-polymerase chain reaction
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- SDS:
-
Sodium dodecyl sulfate
- SDS-PAGE:
-
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SEM:
-
Standard error of the mean
- SPSS:
-
Statistical Product and Service Solutions
- TBS:
-
Tris-buffered saline
- TH:
-
Tyrosine hydroxylase
- TNF-α:
-
Tumor necrosis factor-α
- 6-OHDA:
-
6-Hydroxydopamine
References
Wang G, Pan J, Chen SD (2012) Kinases and kinase signaling pathways: potential therapeutic targets in Parkinson’s disease. Prog Neurobiol 98(2):207–221
Gemma C (2010) Neuroimmunomodulation and aging. Aging Dis 1(3):169–172
Long-Smith CM, Sullivan AM, Nolan YM (2009) The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol 89(3):277–287
Spulber S, Schultzberg M (2010) Connection between inflammatory processes and transmitter function—modulatory effects of interleukin-1. Prog Neurobiol 90(2):256–262
Labandeira-Garcia JL, Rodriguez-Pallares J, Villar-Cheda B, Rodríguez-Perez AI, Garrido-Gil P, Guerra MJ (2011) Aging, angiotensin system and dopaminergic degeneration in the substantia nigra. Aging Dis 2(3):257–274
Decressac M, Volakakis N, Björklund A, Perlmann T (2013) NURR1 in Parkinson disease—from pathogenesis to therapeutic potential. Nat Rev Neurol 9(11):629–636
Lévesque D, Rouillard C (2007) Nur77 and retinoid X receptors: crucial factors in dopamine-related neuroadaptation. Trends Neurosci 30(1):22–30
Nolan YM, Sullivan AM, Toulouse A (2013) Parkinson’s disease in the nuclear age of neuroinflammation. Trends Mol Med 19(3):187–196
Jankovic J, Chen S, Le WD (2005) The role of Nurr1 in the development of dopaminergic neurons and Parkinson’s disease. Prog Neurobiol 77(1-2):128–138
Le WD, Xu P, Jankovic J, Jiang H, Appel SH, Smith RG, Vassilatis DK (2003) Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet 33(1):85–89
Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK (2009) A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 137(1):47–59
Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Björklund A (2012) α-Synuclein-induced down-regulation of Nurr1 disrupts GDNF signaling in nigral dopamine neurons. Sci Transl Med 4(163):163ra156
Cheng Z, Völkers M, Din S, Avitabile D, Khan M, Gude N, Mohsin S, Bo T, Truffa S, Alvarez R, Mason M, Fischer KM, Konstandin MH, Zhang XK, Heller Brown J, Sussman MA (2011) Mitochondrial translocation of Nur77 mediates cardiomyocyte apoptosis. Eur Heart J 32(17):2179–2188
Wang A, Rud J, Olson CMJ, Anguita J, Osborne BA (2009) Phosphorylation of Nur77 by the MEK-ERK-RSK cascade induces mitochondrial translocation and apoptosis in T cells. J Immunol 183(5):3268–3277
Schott J, Reitter S, Philipp J, Haneke K, Schafer H, Stoecklin G (2014) Translational regulation of specific mRNAs controls feedback inhibition and survival during macrophage activation. PLoS Genet 10(6), e1004368
Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, Iwamoto Y, Liao R, Zirlik A, Scherer-Crosbie M, Hedrick CC, Libby P, Nahrendorf M, Weissleder R, Swirski FK (2014) Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res 114(10):1611–1622
Li L, Liu Y, Chen HZ, Li FW, Wu JF, Zhang HK, He JP, Xing YZ, Chen Y, Wang WJ, Tian XY, Li AZ, Zhang Q, Huang PQ, Han J, Lin T, Wu Q (2015) Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nat Chem Biol 11(5):339–346
Papac-Milicevic N, Breuss JM, Zaujec J, Ryban L, Plyushch T, Wagner GA, Fenzl S, Dremsek P, Cabaravdic M, Steiner M, Glass CK, Binder CJ, Uhrin P, Binder BR (2012) The interferon stimulated gene 12 inactivates vasculoprotective functions of NR4A nuclear receptors. Circ Res 110(8):e50–63
Pei L, Castrillo A, Tontonoz P (2006) Regulation of macrophage inflammatory gene expression by the orphan nuclear receptor Nur77. Mol Endocrinol 20(4):786–794
Mohan HM, Aherne CM, Rogers AC, Baird AW, Winter DC, Murphy EP (2012) Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clin Cancer Res 18(12):3223–3228
Lindenboim L, Borner C, Stein R (2011) Nuclear proteins acting on mitochondria. Biochim Biophys Acta 1813(4):584–596
Wang WJ, Wang Y, Chen HZ, Xing YZ, Li FW, Zhang Q, Zhou B, Zhang HK, Zhang J, Bian XL, Li L, Liu Y, Zhao BX, Chen Y, Wu R, Li AZ, Yao LM, Chen P, Zhang Y, Tian XY, Beermann F, Wu M, Han J, Huang PQ, Lin T, Wu Q (2014) Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Nat Chem Biol 10(2):133–140
Kiss B, Toth K, Sarang Z, Garabuczi E, Szondy Z (2015) Retinoids induce Nur77-dependent apoptosis in mouse thymocytes. Biochim Biophys Acta 1853(3):660–670
Lin H, Lin Q, Liu M, Lin Y, Wang X, Chen H, Xia Z, Lu B, Ding F, Wu Q, Wang HR (2014) PKA/Smurf1 signaling-mediated stabilization of Nur77 is required for anticancer drug cisplatin-induced apoptosis. Oncogene 33(13):1629–1639
Aarsland D, Ballard C, Walker Z, Bostrom F, Alves G, Kossakowski K, Leroi I, Pozo-Rodriguez F, Minthon L, Londos E (2009) Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol 8(7):613–618
Emre M, Tsolaki M, Bonuccelli U, Destée A, Tolosa E, Kutzelnigg A, Ceballos-Baumann A, Zdravkovic S, Bladström A, Jones R, Investigators S (2010) Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 9(10):969–977
Montagne A, Hébert M, Jullienne A, Lesept F, Le Béhot A, Louessard M, Gauberti M, Orset C, Ali C, Agin V, Maubert E, Vivien D (2012) Memantine improves safety of thrombolysis for stroke. Stroke 43(10):2774–2781
Wu HM, Tzeng NS, Qian L, Wei SJ, Hu X, Chen SH, Rawls SM, Flood P, Hong JS, Lu RB (2009) Novel neuroprotective mechanisms of memantine: increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology 34(10):2344–2357
Barneda-Zahonero B, Servitja JM, Badiola N, Miñano-Molina AJ, Fadó R, Saura CA, Rodríguez-Alvarez J (2012) Nurr1 protein is required for N-methyl-D-aspartic acid (NMDA) receptor-mediated neuronal survival. J Biol Chem 287(14):11351–11362
Xu YQ, Long L, Yan JQ, Wei L, Pan MQ, Gao HM, Zhou P, Liu M, Zhu CS, Tang BS, Wang Q (2013) Simvastatin induces neuroprotection in 6-OHDA-lesioned PC12 via the PI3K/AKT/caspase 3 pathway and anti-inflammatory responses. CNS Neurosci Ther 19(3):170–177
Du M, Wu M, Fu D, Yang S, Chen J, Wilson K, Lyons TJ (2013) Effects of modified LDL and HDL on retinal pigment epithelial cells: a role in diabetic retinopathy. Diabetologia 56(10):2318–2328
Maijenburg MW, Gilissen C, Melief SM, Kleijer M, Weijer K, Ten Brinke A, Roelofs H, Van Tiel CM, Veltman JA, de Vries CJ, van der Schoot CE, Voermans C (2012) Nuclear receptors Nur77 and Nurr1 modulate mesenchymal stromal cell migration. Stem Cells Dev 21(2):228–238
Sastry L, Johnson T, Hobson MJ, Smucker B, Cornetta K (2002) Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther 9(17):1155–1162
Kaufman AM, Milnerwood AJ, Sepers MD, Coquinco A, She K, Wang L, Lee H, Craig AM, Cynader M, Raymond LA (2012) Opposing roles of synaptic and extrasynaptic NMDA receptor signaling in cocultured striatal and cortical neurons. J Neurosci 32(12):3992–4003
Chung S, Moon JI, Leung A, Aldrich D, Lukianov S, Kitayama Y, Park S, Li Y, Bolshakov VY, Lamonerie T, Kim KS (2011) ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc Natl Acad Sci U S A 108(23):9703–9708
Lee KW, Ma L, Yan X, Liu B, Zhang XK, Cohen P (2005) Rapid apoptosis induction by IGFBP-3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRalpha/Nur77. J Biol Chem 280(17):16942–16948
Rodriguez-Blanco J, Martín V, Herrera F, García-Santos G, Antolín I, Rodriguez C (2008) Intracellular signaling pathways involved in post-mitotic dopaminergic PC12 cell death induced by 6-hydroxydopamine. J Neurochem 107(1):127–140
García-Yagüe ÁJ, Rada P, Rojo AI, Lastres-Becker I, Cuadrado A (2013) Nuclear import and export signals control the subcellular localization of Nurr1 protein in response to oxidative stress. J Biol Chem 288(8):5506–5517
Wroge CM, Hogins J, Eisenman L, Mennerick S (2012) Synaptic NMDA receptors mediate hypoxic excitotoxic death. J Neurosci 32(19):6732–6742
Volbracht C, van Beek J, Zhu C, Blomgren K, Leist M (2006) Neuroprotective properties of memantine in different in vitro and in vivo models of excitotoxicity. Eur J Neurosci 23(10):2611–2622
Hamers AA, Hanna RN, Nowyhed H, Hedrick CC, de Vries CJ (2013) NR4A nuclear receptors in immunity and atherosclerosis. Curr Opin Lipidol 24(5):381–385
Renaud J, Chiasson K, Bournival J, Rouillard C, Martinoli MG (2014) 17β-estradiol delays 6-OHDA-induced apoptosis by acting on Nur77 translocation from the nucleus to the cytoplasm. Neurotox Res 25(1):124–134
Jacobs CM, Boldingh KA, Slagsvold HH, Thoresen GH, Paulsen RE (2004) ERK2 prohibits apoptosis-induced subcellular translocation of orphan nuclear receptor NGFI-B/TR3. J Biol Chem 279:50097–50101
Zhu G, Chen G, Shi L, Feng J, Wang Y, Ye C, Feng W, Niu J, Huang Z (2015) PEGylated rhFGF-2 conveys long-term neuroprotection and improves neuronal function in a rat model of Parkinson’s disease. Mol Neurobiol 51(1):32–42
Basile JR, Binmadi NO, Zhou H, Yang YH, Paoli A, Proia P (2013) Supraphysiological doses of performance enhancing anabolic-androgenic steroids exert direct toxic effects on neuron-like cells. Front Cell Neurosci 7:69
Zhao J, Cheng Y, Yang C, Lau S, Lao L, Shuai B, Cai J, Rong J (2015) Botanical drug puerarin attenuates 6-hydroxydopamine (6-OHDA)-Induced neurotoxicity via upregulating mitochondrial enzyme arginase-2. Mol Neurobiol. In press
Tsai KL, Cheng YY, Leu HB, Lee YY, Chen TJ, Liu DH, Kao CL (2015) Investigating the role of Sirt1-modulated oxidative stress in relation to benign paroxysmal positional vertigo and Parkinson’s disease. Neurobiol Aging 36(9):2607–2616
Huleatt PB, Khoo ML, Chua YY, Tan TW, Liew RS, Balogh B, Deme R, Goloncser F et al (2015) Novel arylalkenylpropargylamines as neuroprotective, potent, and selective monoamine oxidase B inhibitors for the treatment of Parkinson’s disease. J Med Chem 58 (3):1400–1419
Jiang C, Wan X, He Y, Pan T, Jankovic J, Le W (2005) Age-dependent dopaminergic dysfunction in Nurr1 knockout mice. Exp Neurol 191(1):154–162
Besong-Agbo D, Wolf E, Jessen F, Oechsner M, Hametner E, Poewe W, Reindl M, Oertel WH, Noelker C, Bacher M, Dodel R (2013) Naturally occurring α-synuclein autoantibody levels are lower in patients with Parkinson disease. Neurology 80(2):169–175
Liu H, Wei L, Tao Q, Deng H, Ming M, Xu P, Le W (2012) Decreased NURR1 and PITX3 gene expression in Chinese patients with Parkinson’s disease. Eur J Neurol 19(6):870–875
Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG (2011) α-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 10(3):230–240
Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, Li Y, Aro P, Dator R, He C, Hipp MJ, Zabetian CP, Peskind ER, Hu SC, Quinn JF, Galasko DR, Banks WA, Zhang J (2014) Plasma exosomal α-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol 128(5):639–650
Wang Y, Shi M, Chung KA, Zabetian CP, Leverenz JB, Berg D, Srulijes K, Trojanowski JQ, Lee VM, Siderowf AD, Hurtig H, Litvan I, Schiess MC, Peskind ER, Masuda M, Hasegawa M, Lin X, Pan C, Galasko D, Goldstein DS, Jensen PH, Yang H, Cain KC, Zhang J (2012) Phosphorylated α-synuclein in Parkinson’s disease. Sci Transl Med 4(121):121ra120
Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM, Leverenz JB, Montine TJ, Ginghina C, Kang UJ, Cain KC, Wang Y, Aasly J, Goldstein D, Zhang J (2011) Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol 69(3):570–580
Jo AY, Kim MY, Lee HS, Rhee YH, Lee JE, Baek KH, Park CH, Koh HC, Shin I, Lee YS, Lee SH (2009) Generation of dopamine neurons with improved cell survival and phenotype maintenance using a degradation-resistant nurr1 mutant. Stem Cells 27(9):2238–2246
Lin X, Parisiadou L, Sgobio C, Liu G, Yu J, Sun L, Shim H, Gu XL, Luo J, Long CX, Ding J, Mateo Y, Sullivan PH, Wu LG, Goldstein DS, Lovinger D, Cai H (2012) Conditional expression of Parkinson’s disease-related mutant α-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J Neurosci 32(27):9248–9264
Chen HZ, Zhao BX, Zhao WX, Li L, Zhang B, Wu Q (2008) Akt phosphorylates the TR3 orphan receptor and blocks its targeting to the mitochondria. Carcinogenesis 29(11):2078–2088
Volpicelli F, Caiazzo M, Greco D, Consales C, Leone L, Perrone-Capano C, Colucci D’Amato L, di Porzio U (2007) Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons in vitro. J Neurochem 102(2):441–453
Rosi S, Ramirez-Amaya V, Vazdarjanova A, Esparza EE, Larkin PB, Fike JR, Wenk GL, Barnes CA (2009) Accuracy of hippocampal network activity is disrupted by neuroinflammation: rescue by memantine. Brain 132(Pt 9):2464–2477
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant no. 81271427, 81471291), 973 Project (2011CB510000), and Scientific Research Foundation of Guangzhou (grant no. 2014 J4100210), National Natural Science Foundations of Guangdong of China (2014A020212068) to Q.W. Y.X. was partially supported by NIH AT004422 and Vivian L Smith Neurologic Foundation.
Author contributions
X.W., HG, J.Z., Y.X., X.L., and Q.W. conceived and designed the experiments; X.W., H.G., J.Z., Y.X., X.L., J.L., and Q.W. performed the experiments; X.W., J.Z., Y.X., D.C., and Q.W. analyzed the data; Q.W., X.C., L.M., Z.Z., and B.T. contributed reagents/materials/analysis tools; X.C., K.J., Y.X., and Q.W. wrote the paper.
Additional information
Competing financial interests: The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiaobo Wei, Huimin Gao and Jing Zou contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1482 kb)
Rights and permissions
About this article
Cite this article
Wei, X., Gao, H., Zou, J. et al. Contra-directional Coupling of Nur77 and Nurr1 in Neurodegeneration: A Novel Mechanism for Memantine-Induced Anti-inflammation and Anti-mitochondrial Impairment. Mol Neurobiol 53, 5876–5892 (2016). https://doi.org/10.1007/s12035-015-9477-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9477-7