Abstract
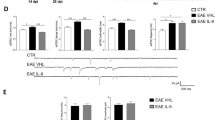
Semaphorin 7A (sema7A) is classified as an immune semaphorin with dual functions in the immune system and in the central nervous system (CNS). These molecules are of interest due to their potential role in multiple sclerosis (MS), which is a chronic demyelinating and neurodegenerative disease of autoimmune origin. In this study, we elucidated the role of sema7A in neuroinflammation using both in vitro and in vivo experimental models. In an in vitro model of neuroinflammation, using cerebellar organotypic slice cultures, we observed that challenge with lipopolysaccharide (LPS) endotoxin did not affect demyelination or cell death in sema7A-deficient cultures compared to wild-type cultures. Moreover, the in vivo outcome of experimental autoimmune encephalomyelitis (EAE) in sema7A-deficient mice was altered in an antigen- and adjuvant-dose-dependent manner, while no differences were observed in the wild-type counterparts. Altogether, these results indicate that sema7A is involved in peripheral immunity and CNS inflammation in MS pathogenesis. Indeed, these data suggest that sema7A might be a potential therapeutic target to treat MS and autoimmune conditions.






Similar content being viewed by others
References
Roth L, Koncina E, Satkauskas S, Cremel G, Aunis D, Bagnard D (2009) The many faces of semaphorins: from development to pathology. Cell Mol Life Sci 66(4):649–666
Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL (2003) Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature 424(6947):398–405
Kopp MA, Brommer B, Gatzemeier N, Schwab JM, Pruss H (2010) Spinal cord injury induces differential expression of the profibrotic semaphorin 7A in the developing and mature glial scar. Glia 58(14):1748–1756
Comeau MR, Johnson R, DuBose RF, Petersen M, Gearing P, Vanden Bos T, Park L, Farrah T et al (1998) A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor, VESPR. Immunity 8(4):473–482
Tamagnone L, Artigiani S, Chen H, He Z, Ming GI, Song H, Chedotal A, Winberg ML et al (1999) Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell 99(1):71–80
Suzuki K, Okuno T, Yamamoto M, Pasterkamp RJ, Takegahara N, Takamatsu H, Kitao T, Takagi J et al (2007) Semaphorin 7A initiates T-cell-mediated inflammatory responses through alpha1beta1 integrin. Nature 446(7136):680–684
Xu X, Ng S, Wu ZL, Nguyen D, Homburger S, Seidel-Dugan C, Ebens A, Luo Y (1998) Human semaphorin K1 is glycosylphosphatidylinositol-linked and defines a new subfamily of viral-related semaphorins. J Biol Chem 273(35):22428–22434
Costa C, Martinez-Saez E, Gutierrez-Franco A, Eixarch H, Castro Z, Ortega-Aznar A, Ramon YCS, Montalban X et al (2015) Expression of semaphorin 3A, semaphorin 7A and their receptors in multiple sclerosis lesions. Mult Scler 21(13):1632–1643
Gutierrez-Franco A, Costa C, Eixarch H, Castillo M, Medina-Rodriguez EM, Bribian A, de Castro F, Montalban X et al (2016) Differential expression of sema3A and sema7A in a murine model of multiple sclerosis: implications for a therapeutic design. Clin Immunol 163:22–33. doi:10.1016/j.clim.2015.12.005
Sato Y, Takahashi H (1998) Molecular cloning and expression of murine homologue of semaphorin K1 gene. Biochim Biophys Acta 1443(3):419–422
Yamada A, Kubo K, Takeshita T, Harashima N, Kawano K, Mine T, Sagawa K, Sugamura K et al (1999) Molecular cloning of a glycosylphosphatidylinositol-anchored molecule CDw108. J Immunol 162(7):4094–4100
Mine T, Harada K, Matsumoto T, Yamana H, Shirouzu K, Itoh K, Yamada A (2000) CDw108 expression during T-cell development. Tissue Antigens 55(5):429–436
Holmes S, Downs AM, Fosberry A, Hayes PD, Michalovich D, Murdoch P, Moores K, Fox J et al (2002) Sema7A is a potent monocyte stimulator. Scand J Immunol 56(3):270–275
Czopik AK, Bynoe MS, Palm N, Raine CS, Medzhitov R (2006) Semaphorin 7A is a negative regulator of T cell responses. Immunity 24(5):591–600
Sospedra M, Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23:683–747
Comabella M, Fernandez M, Martin R, Rivera-Vallve S, Borras E, Chiva C, Julia E, Rovira A et al (2010) Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain 133(Pt 4):1082–1093
Canto E, Tintore M, Villar LM, Borras E, Alvarez-Cermeno JC, Chiva C, Sabido E, Rovira A et al (2014) Validation of semaphorin 7A and ala-beta-his-dipeptidase as biomarkers associated with the conversion from clinically isolated syndrome to multiple sclerosis. J Neuroinflammation 11:181
Mingorance A, Fontana X, Sole M, Burgaya F, Urena JM, Teng FY, Tang BL, Hunt D et al (2004) Regulation of Nogo and Nogo receptor during the development of the entorhino-hippocampal pathway and after adult hippocampal lesions. Mol Cell Neurosci 26(1):34–49. doi:10.1016/j.mcn.2004.01.001
Bribian A, Nocentini S, Llorens F, Gil V, Mire E, Reginensi D, Yoshida Y, Mann F et al (2014) Sema3E/PlexinD1 regulates the migration of hem-derived Cajal-Retzius cells in developing cerebral cortex. Nat Commun 5:4265. doi:10.1038/ncomms5265
Pasterkamp RJ, Kolk SM, Hellemons AJ, Kolodkin AL (2007) Expression patterns of semaphorin7A and plexinC1 during rat neural development suggest roles in axon guidance and neuronal migration. BMC Dev Biol 7:98. doi:10.1186/1471-213X-7-98
Mingorance A, Fontana X, Soriano E, Del Rio JA (2005) Overexpression of myelin-associated glycoprotein after axotomy of the perforant pathway. Mol Cell Neurosci 29(3):471–483. doi:10.1016/j.mcn.2005.03.016
Soriano E, Alvarado-Mallart RM, Dumesnil N, Del Rio JA, Sotelo C (1997) Cajal-Retzius cells regulate the radial glia phenotype in the adult and developing cerebellum and alter granule cell migration. Neuron 18(4):563–577
di Penta A, Moreno B, Reix S, Fernandez-Diez B, Villanueva M, Errea O, Escala N, Vandenbroeck K et al (2013) Oxidative stress and proinflammatory cytokines contribute to demyelination and axonal damage in a cerebellar culture model of neuroinflammation. PLoS One 8(2):e54722. doi:10.1371/journal.pone.0054722
Gil V, Bichler Z, Lee JK, Seira O, Llorens F, Bribian A, Morales R, Claverol-Tinture E et al (2010) Developmental expression of the oligodendrocyte myelin glycoprotein in the mouse telencephalon. Cereb Cortex 20(8):1769–1779. doi:10.1093/cercor/bhp246
Magalon K, Zimmer C, Cayre M, Khaldi J, Bourbon C, Robles I, Tardif G, Viola A et al (2012) Olesoxime accelerates myelination and promotes repair in models of demyelination. Ann Neurol 71(2):213–226. doi:10.1002/ana.22593
Espejo C, Carrasco J, Hidalgo J, Penkowa M, Garcia A, Saez-Torres I, Martinez-Caceres EM (2001) Differential expression of metallothioneins in the CNS of mice with experimental autoimmune encephalomyelitis. Neuroscience 105(4):1055–1065
Baker D, Amor S (2012) Publication guidelines for refereeing and reporting on animal use in experimental autoimmune encephalomyelitis. J Neuroimmunol 242(1–2):78–83
Eixarch H, Gutierrez-Franco A, Montalban X, Espejo C (2013) Semaphorins 3A and 7A: potential immune and neuroregenerative targets in multiple sclerosis. Trends Mol Med 19(3):157–164. doi:10.1016/j.molmed.2013.01.003
Kim CW, Cho EH, Lee YJ, Kim YH, Hah YS, Kim DR (2006) Disease-specific proteins from rheumatoid arthritis patients. J Korean Med Sci 21(3):478–484
Reilkoff RA, Peng H, Murray LA, Peng X, Russell T, Montgomery R, Feghali-Bostwick C, Shaw A et al (2013) Semaphorin 7a + regulatory T cells are associated with progressive idiopathic pulmonary fibrosis and are implicated in transforming growth factor-beta1-induced pulmonary fibrosis. Am J Respir Crit Care Med 187(2):180–188. doi:10.1164/rccm.201206-1109OC
deLuca LE, Pikor NB, O’Leary J, Galicia-Rosas G, Ward LA, Defreitas D, Finlay TM, Ousman SS et al (2010) Substrain differences reveal novel disease-modifying gene candidates that alter the clinical course of a rodent model of multiple sclerosis. J Immunol 184(6):3174–3185
Pinto LH, Eaton E, Chen B, Fleisher J, Shuster D, McCauley J, Kedainis D, Siepka SM et al (2008) Gene-environment interactions in a mutant mouse kindred with native airway constrictor hyperresponsiveness. Mamm Genome 19(1):2–14
Acknowledgments
We thank Prof. A. Kolodkin for kindly providing the sema7A-KO mice, D. Johnson for helping with the transfer of the animals and assistance with genotyping and Prof. R. J Pasterkamp for providing the sema7A and Plexin-C1 cDNA probes. This project was supported by the Fondo de Investigación Sanitaria (FIS; Instituto de Salud Carlos III, Ministry of Economy and Competitiveness in Spain; PI12/02144, granted to CE) and “Red Española de Esclerosis Múltiple (REEM)” (RD12/0032) which is sponsored by the FIS, and the “Ajuts per donar Suport als Grups de Recerca de Catalunya” (2014 SGR 1082) which is sponsored by the “Agència de Gestió d’Ajuts Universitaris i de Recerca” (AGAUR; Generalitat de Catalunya). It was also supported by grants from the Spanish Ministry of Economy and Competitiveness (MINECO) (BFU2012-32617 and BFU2015-67777-R), the Spanish prion network (Prionet Spain, AGL2015-71764-REDT), CIBERNED (PI2014/02-4 and PRY-14-114), La Caixa Obra Social Foundation (P1-L14) and La Marató de TV3 (20143410) granted to JADR. AG-F was supported by the PFIS programme (FI10/00456), HE was supported by the “Sara Borrell” programme (CD09/00363) and CE is supported by the “Miguel Servet” programme (CP13/00028), all of which are under the FIS. VG is supported by a Juan de la Cierva post-doctoral fellowship of MINECO (JCI-2012-14356).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Research Involving Animals
All experiments were performed in strict accordance with EU (Directive 2010/63/UE) and Spanish regulations (Real Decreto 53/2013; Generalitat de Catalunya Decret 214/97). The Ethics Committee on Animal Experimentation of the Vall d’Hebron Research Institute and the University of Barcelona approved all procedures described in this study (protocol number: 39/09 CEEA–DAAM 5612, and 141/15 and 329/14 respectively).
Disclosure of Potential Conflict of Interest
AGF, HE, CC, MC, VG, LCB, JADR, CE declare no financial conflict of interest.
XM has received speaking honoraria and travel expenses for scientific meetings, has been a steering committee member of clinical trials or participated in advisory boards of clinical trials in the past with Bayer Schering Pharma, Biogen Idec, EMD Merck Serono, Genentech, Genzyme, Novartis, Sanofi-Aventis, Teva Pharmaceuticals and Almirall.
Additional information
Ana Gutiérrez-Franco and Herena Eixarch contributed equally to this work.
Electronic supplementary material
ESM 1
(PDF 442 kb)
Rights and permissions
About this article
Cite this article
Gutiérrez-Franco, A., Eixarch, H., Costa, C. et al. Semaphorin 7A as a Potential Therapeutic Target for Multiple Sclerosis. Mol Neurobiol 54, 4820–4831 (2017). https://doi.org/10.1007/s12035-016-0154-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0154-2