Abstract
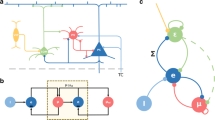
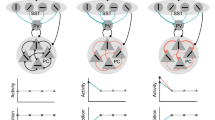
The embodied mammalian brain evolved to adapt to an only partially known and knowable world. The adaptive labeling of the world is critically dependent on the neocortex which in turn is modulated by a range of subcortical systems such as the thalamus, ventral striatum, and the amygdala. A particular case in point is the learning paradigm of classical conditioning where acquired representations of states of the world such as sounds and visual features are associated with predefined discrete behavioral responses such as eye blinks and freezing. Learning progresses in a very specific order, where the animal first identifies the features of the task that are predictive of a motivational state and then forms the association of the current sensory state with a particular action and shapes this action to the specific contingency. This adaptive feature selection has both attentional and memory components, i.e., a behaviorally relevant state must be detected while its representation must be stabilized to allow its interfacing to output systems. Here, we present a computational model of the neocortical systems that underlie this feature detection process and its state-dependent modulation mediated by the amygdala and its downstream target the nucleus basalis of Meynert. In particular, we analyze the role of different populations of inhibitory interneurons in the regulation of cortical activity and their state-dependent gating of sensory signals. In our model, we show that the neuromodulator acetylcholine (ACh), which is in turn under control of the amygdala, plays a distinct role in the dynamics of each population and their associated gating function serving the detection of novel sensory features not captured in the state of the network, facilitating the adjustment of cortical sensory representations and regulating the switching between modes of attention and learning.




Similar content being viewed by others
References
Turchi J, Sarter M (1997) Cortical acetylcholine and processing capacity: effects of cortical cholinergic deafferentation on crossmodal divided attention in rats. Cogn Brain Res 6:147–158
Himmelheber AM, Sarter M, Bruno JP (2000) Increases in cortical acetylcholine release during sustained attention performance in rats. Cogn Brain Res 9:313–325
Klinkenberg I, Sambeth A, Blokland A (2011) Acetylcholine and attention. Behav Brain Res 221:430–442
Phillis JW (1968) Acetylcholine release from the cerebral cortex: its role in cortical arousal. Brain Res 7:378–389
Gould R, Nedelcovych M, Dencker D et al (2014) Influence of M1 muscarinic acetylcholine receptor activation on arousal and cognitive performance using electroencephalography and novel touchscreen cognition assessment (845.5). FASEB J 28:845
Jones BE, Cuello AC (1989) Afferents to the basal forebrain cholinergic cell area from pontomesencephalic—catecholamine, serotonin, and acetylcholine—neurons. Neuroscience 31:37–61. doi:10.1016/0306-4522(89)90029-8
Smiley JF, Subramanian M, Mesulam M-M (1999) Monoaminergic–cholinergic interactions in the primate basal forebrain. Neuroscience 93:817–829. doi:10.1016/S0306-4522(99)00116-5
Zaborszky L (1989) Afferent connections of the forebrain cholinergic projection neurons, with special reference to monoaminergic and peptidergic fibers. In: Central cholinergic synaptic transmission. Birkhäuser Basel, pp 12–32. doi:10.1007/978-3-0348-9138-7_2
Carnes KM, Fuller TA, Price JL (1990) Sources of presumptive glutamatergic/aspartatergic afferents to the magnocellular basal forebrain in the rat. J Comp Neurol 302:824–852. doi:10.1002/cne.903020413
Zaborszky L, Gaykema R, Swanson D, Cullinan W (1997) Cortical input to the basal forebrain. Neuroscience 79:1051–1078. doi:10.1016/S0306-4522(97)00049-3
Gastard M, Jensen SL, Martin JR III et al (2002) The caudal sublenticular region/anterior amygdaloid area is the only part of the rat forebrain and mesopontine tegmentum occupied by magnocellular cholinergic neurons that receives outputs from the central division of extended amygdala. Brain Res 957:207–222. doi:10.1016/S0006-8993(02)03513-8
Grove EA (1988) Neural associations of the substantia innominata in the rat: afferent connections. J Comp Neurol 277:315–346. doi:10.1002/cne.902770302
Liu AKL, Chang RC-C, Pearce RKB, Gentleman SM (2015) Nucleus basalis of Meynert revisited: anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol 129:527–540. doi:10.1007/s00401-015-1392-5
Kawaguchi Y (1997) Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol 78:1743–1747. doi:10.1016/0006-8993(82)90067-1
Yi F, Ball J, Stoll KE et al (2014) Direct excitation of parvalbumin-positive interneurons by M1 muscarinic acetylcholine receptors: roles in cellular excitability, inhibitory transmission and cognition. J Physiol 592:3463–3494. doi:10.1113/jphysiol.2014.275453
Lawrence JJ (2008) Cholinergic control of GABA release: emerging parallels between neocortex and hippocampus. Trends Neurosci 31:317–327. doi:10.1016/j.tins.2008.03.008
Markram H, Toledo-Rodriguez M, Wang Y et al (2004) Interneurons of the neocortical inhibitory system. Nat Rev Neurosci 5:793–807. doi:10.1038/nrn1519
Connors BW, Gutnick MJ (1990) Intrinsic firing patterns of diverse neocortical neurons. Trends Neurosci 13:99–104
Kawaguchi Y, Kubota Y (1997) GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 7:476–486. doi:10.1093/cercor/7.6.476
Kawaguchi Y (1993) Groupings of nonpyramidal and pyramidal cells with specific physiological and morphological characteristics in rat frontal cortex. J Neurophysiol 69:416–431
Kubota Y, Hattori R, Yui Y (1994) Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res 649:159–173
Shepherd GM (2003) The synaptic organization of the brain. Oxford University Press, New York
Rudy B, Fishell G, Lee S, Hjerling-leffler J (2010) Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71(1):45–61. doi:10.1002/dneu.20853
Disney AA, Aoki C, Hawken MJ (2007) Gain modulation by nicotine in macaque V1. Neuron 56:701–713. doi:10.1016/j.neuron.2007.09.034
Disney AA, Aoki C (2008) Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin-immunoreactive neurons. J Comp Neurol 507:1748–1762
Disney AA, Alasady HA, Reynolds JH (2014) Muscarinic acetylcholine receptors are expressed by most parvalbumin-immunoreactive neurons in area MT of the macaque. Brain Behav 4:431–445. doi:10.1002/brb3.225
Demars MP, Morishita H (2014) Cortical parvalbumin and somatostatin GABA neurons express distinct endogenous modulators of nicotinic acetylcholine receptors. Mol Brain 7:75. doi:10.1186/s13041-014-0075-9
Markov NT, Misery P, Falchier A et al (2011) Weight consistency specifies regularities of macaque cortical networks. Cereb Cortex 21:1254–1272. doi:10.1093/cercor/bhq201
Benucci A, Verschure PFMJ, König P (2007) Dynamical features of higher-order correlation events: Impact on cortical cells. Cogn Neurodyn. doi:10.1007/s11571-006-9000-y
Markov NT, Ercsey-Ravasz MM, Gomes ARR et al (2012) A weighted and directed interareal connectivity matrix for macaque cerebral cortex. Cereb Cortex bhs270
Puigbò J-Y, van Wijngaarden J, Low SC, Verschure PFMJ (2016) Synaptogenesis: constraining synaptic plasticity based on a distance rule. In: Proc. of the Int. Conf. Artif. Neural Networks in LNCS 9886:28–35. doi:10.1007/978-3-319-44778-0_4
Packer AM, Yuste R (2011) Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci 31:13260–13271
Adesnik H, Bruns W, Taniguchi H (2012) A neural circuit for spatial summation in visual cortex. Nature 490:226–231. doi:10.1038/nature11526
Gulledge AT (2005) Cholinergic inhibition of neocortical pyramidal neurons. J Neurosci 25:10308–10320. doi:10.1523/JNEUROSCI.2697-05.2005
Douglas RJ, Martin K a C (2007) Mapping the matrix: the ways of neocortex. Neuron 56:226–238. doi:10.1016/j.neuron.2007.10.017
Yang X, Wang K, Shamma SA (1992) Auditory representations of acoustic signals. IEEE Trans Inf Theory 38:824–839
Carandini M, Heeger DJ, Movshon JA (1997) Linearity and normalization in simple cells of the macaque primary visual cortex. J Neurosci 17:8621–8644
Hasselmo ME (2006) The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16:710–715. doi:10.1016/j.conb.2006.09.002
Metherate R, Cox CL, Ashe JH (1992) Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci 12:4701–4711
Lee JH, Whittington MA, Kopell NJ (2015) Potential mechanisms underlying intercortical signal regulation via cholinergic neuromodulators. J Neurosci 35:15000–15014. doi:10.1523/JNEUROSCI.0629-15.2015
Callaway EM (2004) Feedforward, feedback and inhibitory connections in primate visual cortex. Neural Netw 17:625–632. doi:10.1016/j.neunet.2004.04.004
Bastos AM, Vezoli J, Bosman CA et al (2015) Visual areas exert feedforward and feedback influences through distinct frequency channels. Neuron 85:390–401. doi:10.1016/j.neuron.2014.12.018
Michalareas G, Vezoli J, Van Pelt S et al (2016) Alpha-beta and gamma rhythms subserve feedback and feedforward influences among human visual cortical areas. Neuron 89:384–397. doi:10.1016/j.neuron.2015.12.018
Gross J (2016) Let the rhythm guide you: non-invasive tracking of cortical communication channels. Neuron 89:247. doi:10.1016/j.neuron.2016.01.001
Froemke RC, Carcea I, Barker AJ et al (2013) Long-term modification of cortical synapses improves sensory perception. Nat Neurosci 16:79–88. doi:10.1038/nn.3274
Weinberger NM (2004) Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci 5:279–290. doi:10.1038/nrn1366
Yu AJ, Dayan P (2005) Uncertainty, neuromodulation, and attention. Neuron 46:681–692. doi:10.1016/j.neuron.2005.04.026
Roopun AK, Lebeau FEN, Rammell J et al (2010) Cholinergic neuromodulation controls directed temporal communication in neocortex in vitro. Front Neural Circuits 4:8. doi:10.3389/fncir.2010.00008
Benna MK, Fusi S (2016) Computational principles of synaptic memory consolidation. Nat Neurosci 19:1697–1706. doi:10.1038/nn.4401
Aosaki T, Miura M, Suzuki T et al (2010) Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int. doi:10.1111/j.1447-0594.2010.00588.x
Lalumiere RT, Mcgaugh JL (2005) Memory enhancement induced by post-training intrabasolateral amygdala infusions of β-adrenergic or muscarinic agonists requires activation of dopamine receptors: involvement of right, but not left, basolateral amygdala. Learn Mem 12:527–532. doi:10.1101/lm.97405
Acknowledgments
This work has been supported by the European Research Council’s CDAC project: “The Role of Consciousness in Adaptive Behavior: A Combined Empirical, Computational and Robot based Approach” (ERC-2013- ADG 341196); as well as the European Project What You Say Is What You Did WYSIWYD (FP7 ICT 612139).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Puigbò, JY., Maffei, G., Herreros, I. et al. Cholinergic Behavior State-Dependent Mechanisms of Neocortical Gain Control: a Neurocomputational Study. Mol Neurobiol 55, 249–257 (2018). https://doi.org/10.1007/s12035-017-0737-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-017-0737-6