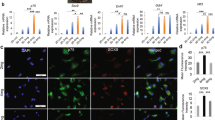
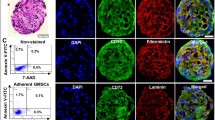
Abstract
Non-genetic induction of somatic cells into neural crest stem-like cells (NCSCs) is promising for potential cell-based therapies for post-traumatic peripheral nerve regeneration. Here, we report that human gingiva-derived mesenchymal stem cells (GMSCs) could be reproducibly and readily induced into NCSCs via non-genetic approaches. Compared to parental GMSCs, induced NCSC population had increased expression in NCSC-related genes and displayed robust differentiation into neuronal and Schwann-like cells. Knockdown of the expression of Yes-associated protein 1 (YAP1), a critical mechanosensor and mechanotransducer, attenuated the expression of NCSC-related genes; specific blocking of RhoA/ROCK activity and non-muscle myosin II (NM II)-dependent contraction suppressed YAP1 and NCSC-related genes and concurrently abolished neural spheroid formation in NCSCs. Using a rat model of facial nerve defect, implantation of NCSC-laden nerve conduits promoted functional regeneration of the injured nerve. These promising findings demonstrate that induced NCSCs derived from GMSCs represent an easily accessible and promising source of neural stem-like cells for peripheral nerve regeneration.










Similar content being viewed by others
References
Trounson A, McDonald C (2015) Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell 17(1):11–22. https://doi.org/10.1016/j.stem.2015.06.007
Murphy SV, Atala A (2014) 3D bioprinting of tissues and organs. Nat Biotechnol 32(8):773–785. https://doi.org/10.1038/nbt.2958
Mandrycky C, Wang Z, Kim K et al. (2015) 3D bioprinting for engineering complex tissues. Biotechnol Adv
Hsieh FY, Hsu SH (2015) 3D bioprinting: a new insight into the therapeutic strategy of neural tissue regeneration. Organogenesis 0
Sensharma P, Madhumathi G, Jayant RD, Jaiswal AK (2017) Biomaterials and cells for neural tissue engineering: current choices. Mater Sci Eng C Mater Biol Appl 77:1302–1315. https://doi.org/10.1016/j.msec.2017.03.264
Takahashi H, Itoga K, Shimizu T, Yamato M, Okano T (2016) Human neural tissue construct fabrication based on scaffold-free tissue engineering. Adv Healthcare Mater 5(15):1931–1938. https://doi.org/10.1002/adhm.201600197
Euler de Souza Lucena E, Guzen FP, Lopes de Paiva Cavalcanti JR, Galvão Barboza CA, Silva do Nascimento Júnior E, Cavalcante JS (2014) Experimental considerations concerning the use of stem cells and tissue engineering for facial nerve regeneration: a systematic review. J Oral Maxillofac Surg 72(5):1001–1012. https://doi.org/10.1016/j.joms.2013.11.006
Gu X, Ding F, Williams DF (2014) Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 35(24):6143–6156. https://doi.org/10.1016/j.biomaterials.2014.04.064
Bhangra KS, Busuttil F, Phillips JB et al (2016) Using stem cells to grow artificial tissue for peripheral nerve repair. Stem Cells Int 2016:7502178
Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27(3):275–280. https://doi.org/10.1038/nbt.1529
Picard-Riera N, Nait-Oumesmar B, Baron-Van EA (2004) Endogenous adult neural stem cells: limits and potential to repair the injured central nervous system. J Neurosci Res 76(2):223–231. https://doi.org/10.1002/jnr.20040
Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K et al (2011) Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A 108(19):7838–7843. https://doi.org/10.1073/pnas.1103113108
Lujan E, Chanda S, Ahlenius H, Sudhof TC, Wernig M (2012) Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A 109(7):2527–2532. https://doi.org/10.1073/pnas.1121003109
Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G et al (2012) Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10(4):465–472. https://doi.org/10.1016/j.stem.2012.02.021
Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR et al (2012) Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11(1):100–109. https://doi.org/10.1016/j.stem.2012.05.018
Thier M, Worsdorfer P, Lakes YB et al (2012) Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10(4):473–479. https://doi.org/10.1016/j.stem.2012.03.003
Kim YJ, Lim H, Li Z, Oh Y, Kovlyagina I, Choi IY, Dong X, Lee G (2014) Generation of multipotent induced neural crest by direct reprogramming of human postnatal fibroblasts with a single transcription factor. Cell Stem Cell 15(4):497–506. https://doi.org/10.1016/j.stem.2014.07.013
Yu KR, Shin JH, Kim JJ et al. (2015) Rapid and efficient direct conversion of human adult somatic cells into neural stem cells by HMGA2/let-7b. Cell Rep
Su G, Zhao Y, Wei J, Xiao Z, Chen B, Han J, Chen L, Guan J et al (2013) Direct conversion of fibroblasts into neural progenitor-like cells by forced growth into 3D spheres on low attachment surfaces. Biomaterials 34(24):5897–5906. https://doi.org/10.1016/j.biomaterials.2013.04.040
Feng N, Han Q, Li J, Wang S, Li H, Yao X, Zhao RC (2014) Generation of highly purified neural stem cells from human adipose-derived mesenchymal stem cells by Sox1 activation. Stem Cells Dev 23(5):515–529. https://doi.org/10.1089/scd.2013.0263
Cheng L, Hu W, Qiu B, Zhao J, Yu Y, Guan W, Wang M, Yang W et al (2014) Generation of neural progenitor cells by chemical cocktails and hypoxia. Cell Res 24(6):665–679. https://doi.org/10.1038/cr.2014.32
Dupin E, Coelho-Aguiar JM (2013) Isolation and differentiation properties of neural crest stem cells. Cytometry A 83:38–47
Kaltschmidt B, Kaltschmidt C, Widera D (2012) Adult craniofacial stem cells: sources and relation to the neural crest. Stem Cell Rev 8(3):658–671. https://doi.org/10.1007/s12015-011-9340-9
Boddupally K, Wang G, Chen Y, Kobielak A (2016) Lgr5 marks neural crest derived multipotent oral stromal stem cells. Stem Cells 34(3):720–731. https://doi.org/10.1002/stem.2314
Abe S, Yamaguchi S, Sato Y, Harada K (2016) Sphere-derived multipotent progenitor cells obtained from human oral mucosa are enriched in neural crest cells. Stem Cells Transl Med 5(1):117–128. https://doi.org/10.5966/sctm.2015-0111
Xu X, Chen C, Akiyama K, Chai Y, AD LE, Wang Z, Shi S (2013) Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J Dent Res 92(9):825–832. https://doi.org/10.1177/0022034513497961
Fournier BP, Loison-Robert LS, Ferre FC et al (2016) Characterisation of human gingival neural crest-derived stem cells in monolayer and neurosphere cultures. Eur Cell Mater. 31:40–58. https://doi.org/10.22203/eCM.v031a04
Morikawa S, Ouchi T, Shibata S et al (2016) Applications of mesenchymal stem cells and neural crest cells in craniofacial skeletal research. Stem Cells Int 2016:2849879
Wislet-Gendebien S, Laudet E, Neirinckx V, Alix P, Leprince P, Glejzer A, Poulet C, Hennuy B et al (2012) Mesenchymal stem cells and neural crest stem cells from adult bone marrow: characterization of their surprising similarities and differences. Cell Mol Life Sci 69(15):2593–2608. https://doi.org/10.1007/s00018-012-0937-1
Coste C, Neirinckx V, Sharma A, Agirman G, Rogister B, Foguenne J, Lallemend F, Gothot A et al (2017) Human bone marrow harbors cells with neural crest-associated characteristics like human adipose and dermis tissues. PLoS One 12(7):e0177962. https://doi.org/10.1371/journal.pone.0177962
Tseng TC, Hsieh FY, Dai NT, Hsu S (2016) Substrate-mediated reprogramming of human fibroblasts into neural crest stem-like cells and their applications in neural repair. Biomaterials 102:148–161. https://doi.org/10.1016/j.biomaterials.2016.06.020
La Noce M, Mele L, Tirino V et al (2014) Neural crest stem cell population in craniomaxillofacial development and tissue repair. Eur Cell Mater 28:348–357. https://doi.org/10.22203/eCM.v028a24
Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, AD LE (2009) Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol 183(12):7787–7798. https://doi.org/10.4049/jimmunol.0902318
Zhang Q, Nguyen P, Xu Q, Park W, Lee S, Furuhashi A, AD LE (2017) Neural progenitor-like cells induced from human gingiva-derived mesenchymal stem cells regulate myelination of Schwann cells in rat sciatic nerve regeneration. Stem Cells Transl Med 6(2):458–470. https://doi.org/10.5966/sctm.2016-0177
Peltier J, Ormerod BK, Schaffer DV (2010) Isolation of adult hippocampal neural progenitors. Methods Mol Biol 621:57–63. https://doi.org/10.1007/978-1-60761-063-2_4
Siebzehnrubl FA, Steindler DA (2013) Isolating and culturing of precursor cells from the adult human brain. Methods Mol Biol 1059:79–86. https://doi.org/10.1007/978-1-62703-574-3_7
Struckhoff AP, Del Valle L (2013) Neurospheres and glial cell cultures: immunocytochemistry for cell phenotyping. Methods Mol Biol 1078:119–132. https://doi.org/10.1007/978-1-62703-640-5_10
Bottenstein JE, Sato GH (1979) Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A 76(1):514–517. https://doi.org/10.1073/pnas.76.1.514
Barnes D, Sato G (1980) Serum-free cell culture: a unifying approach. Cell 22(3):649–655. https://doi.org/10.1016/0092-8674(80)90540-1
Tomita K, Madura T, Sakai Y, Yano K, Terenghi G, Hosokawa K (2013) Glial differentiation of human adipose-derived stem cells: implications for cell-based transplantation therapy. Neuroscience 236:55–65. https://doi.org/10.1016/j.neuroscience.2012.12.066
Martens W, Sanen K, Georgiou M, Struys T, Bronckaers A, Ameloot M, Phillips J, Lambrichts I (2014) Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J 28(4):1634–1643. https://doi.org/10.1096/fj.13-243980
Cao J, Xiao Z, Jin W, Chen B, Meng D, Ding W, Han S, Hou X et al (2013) Induction of rat facial nerve regeneration by functional collagen scaffolds. Biomaterials 34(4):1302–1310. https://doi.org/10.1016/j.biomaterials.2012.10.031
Sasaki R, Matsumine H, Watanabe Y, Takeuchi Y, Yamato M, Okano T, Miyata M, Ando T (2014) Electrophysiologic and functional evaluations of regenerated facial nerve defects with a tube containing dental pulp cells in rats. Plast Reconstr Surg 134(5):970–978. https://doi.org/10.1097/PRS.0000000000000602
Matsumine H, Takeuchi Y, Sasaki R, Kazama T, Kano K, Matsumoto T, Sakurai H, Miyata M et al (2014) Adipocyte-derived and dedifferentiated fat cells promoting facial nerve regeneration in a rat model. Plast Reconstr Surg 134(4):686–697. https://doi.org/10.1097/PRS.0000000000000537
Degistirici O, Jaquiery C, Schonebeck B et al (2008) Defining properties of neural crest-derived progenitor cells from the apex of human developing tooth. Tissue Eng A 14(2):317–330. https://doi.org/10.1089/tea.2007.0221
Davies LC, Locke M, Webb RD et al (2010) A multipotent neural crest-derived progenitor cell population is resident within the oral mucosa lamina propria. Stem Cells Dev 19(6):819–830. https://doi.org/10.1089/scd.2009.0089
Toma JG, McKenzie IA, Bagli D, Miller FD (2005) Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells 23(6):727–737. https://doi.org/10.1634/stemcells.2004-0134
Nagoshi N, Shibata S, Kubota Y, Nakamura M, Nagai Y, Satoh E, Morikawa S, Okada Y et al (2008) Ontogeny and multipotency of neural crest-derived stem cells in mouse bone marrow, dorsal root ganglia, and whisker pad. Cell Stem Cell 2(4):392–403. https://doi.org/10.1016/j.stem.2008.03.005
Menendez L, Yatskievych TA, Antin PB, Dalton S (2011) Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci U S A 108(48):19240–19245. https://doi.org/10.1073/pnas.1113746108
Halder G, Dupont S, Piccolo S (2012) Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol 13(9):591–600. https://doi.org/10.1038/nrm3416
Serinagaoglu Y, Pare J, Giovannini M et al (2015) Nf2-Yap signaling controls the expansion of DRG progenitors and glia during DRG development. Dev Biol 398(1):97–109. https://doi.org/10.1016/j.ydbio.2014.11.017
Ding R, Weynans K, Bossing T et al. (2016) The Hippo signalling pathway maintains quiescence in Drosophila neural stem cells. Nat Commun 7
Hindley CJ, Condurat AL, Menon V, Thomas R, Azmitia LM, Davis JA, Pruszak J (2016) The hippo pathway member YAP enhances human neural crest cell fate and migration. Sci Rep 6(1):23208. https://doi.org/10.1038/srep23208
Yee HF Jr, Melton AC, Tran BN (2001) RhoA/rho-associated kinase mediates fibroblast contractile force generation. Biochem Biophys Res Commun 280(5):1340–1345. https://doi.org/10.1006/bbrc.2001.4291
Sandquist JC, Swenson KI, Demali KA et al (2006) Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem 281(47):35873–35883. https://doi.org/10.1074/jbc.M605343200
Kovacs M, Toth J, Hetenyi C et al (2004) Mechanism of blebbistatin inhibition of myosin II. J Biol Chem 279(34):35557–35563. https://doi.org/10.1074/jbc.M405319200
Clewes O, Narytnyk A, Gillinder KR, Loughney AD, Murdoch AP, Sieber-Blum M (2011) Human epidermal neural crest stem cells (hEPI-NCSC)—characterization and directed differentiation into osteocytes and melanocytes. Stem Cell Rev 7(4):799–814. https://doi.org/10.1007/s12015-011-9255-5
Biernaskie J (2010) Human hair follicles: “bulging” with neural crest-like stem cells. J Investig Dermatol 130(5):1202–1204. https://doi.org/10.1038/jid.2009.449
Krejci E, Grim M (2010) Isolation and characterization of neural crest stem cells from adult human hair follicles. Folia Biol 56(4):149–157
Hara S, Hayashi R, Soma T, Kageyama T, Duncan T, Tsujikawa M, Nishida K (2014) Identification and potential application of human corneal endothelial progenitor cells. Stem Cells Dev 23(18):2190–2201. https://doi.org/10.1089/scd.2013.0387
Hauser S, Widera D, Qunneis F, Müller J, Zander C, Greiner J, Strauss C, Lüningschrör P et al (2012) Isolation of novel multipotent neural crest-derived stem cells from adult human inferior turbinate. Stem Cells Dev 21(5):742–756. https://doi.org/10.1089/scd.2011.0419
Pelaez D, Huang CY, Cheung HS (2013) Isolation of pluripotent neural crest-derived stem cells from adult human tissues by connexin-43 enrichment. Stem Cells Dev 22(21):2906–2914. https://doi.org/10.1089/scd.2013.0090
Yoo J, Noh M, Kim H, Jeon NL, Kim BS, Kim J (2015) Nanogrooved substrate promotes direct lineage reprogramming of fibroblasts to functional induced dopaminergic neurons. Biomaterials 45:36–45. https://doi.org/10.1016/j.biomaterials.2014.12.049
Hsu SH, Huang GS, Lin SY et al (2012) Enhanced chondrogenic differentiation potential of human gingival fibroblasts by spheroid formation on chitosan membranes. Tissue Eng A 18(1-2):67–79. https://doi.org/10.1089/ten.tea.2011.0157
Huang GS, Dai LG, Yen BL, Hsu S (2011) Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 32(29):6929–6945. https://doi.org/10.1016/j.biomaterials.2011.05.092
Boyle ST, Samuel MS (2016) Mechano-reciprocity is maintained between physiological boundaries by tuning signal flux through the Rho-associated protein kinase. Small GTPases 7(3):139–146. https://doi.org/10.1080/21541248.2016.1173771
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P et al (2013) Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15(6):637–646. https://doi.org/10.1038/ncb2756
Volk GF, Pantel M, Guntinas-Lichius O (2010) Modern concepts in facial nerve reconstruction. Head Face Med 6(1):25. https://doi.org/10.1186/1746-160X-6-25
Kingham PJ, Kolar MK, Novikova LN, Novikov LN, Wiberg M (2014) Stimulating the neurotrophic and angiogenic properties of human adipose-derived stem cells enhances nerve repair. Stem Cells Dev 23(7):741–754. https://doi.org/10.1089/scd.2013.0396
Guo ZY, Sun X, Xu XL, Zhao Q, Peng J, Wang Y (2015) Human umbilical cord mesenchymal stem cells promote peripheral nerve repair via paracrine mechanisms. Neural Regen Res 10(4):651–658. https://doi.org/10.4103/1673-5374.155442
Gervois P, Struys T, Hilkens P, Bronckaers A, Ratajczak J, Politis C, Brône B, Lambrichts I et al (2015) Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells Dev 24(3):296–311. https://doi.org/10.1089/scd.2014.0117
Yamamoto T, Osako Y, Ito M et al. (2015) Trophic effects of dental pulp stem cells on Schwann Cells in peripheral nerve regeneration. cell transplantation
Funding
This work was supported by the National Institute of Health Research Grant, R01DE 019932 (to A. L.), the Osteo Science Foundation (OSF) (to Q. Z. Z., and A. L.), Oral and Maxillofacial Surgery Foundation (OMSF) Research Support Grant (to Q. Z. Z. and A. L.), the Schoenleber Funding Support (A. L.), and the US Department of Defense, W81XWH-16-1-0796 & W81XWH-15-1-0466 (to D. K. C.).
Author information
Authors and Affiliations
Contributions
Qunzhou Zhang: Conception and Design, Collection and/or assembly of data, Data analysis and interpretation, Manuscript writing. Phuong D. Nguyen: Collection and/or assembly of data. Shihong Shi: Collection and/or assembly of data. Justin C. Burrell: Collection and/or assembly of data. Kacy D. Cullen: Conception and Design, Manuscript writing. Anh D. Le: Conception and Design, Manuscript writing, Final approval of manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, Q., Nguyen, P.D., Shi, S. et al. Neural Crest Stem-Like Cells Non-genetically Induced from Human Gingiva-Derived Mesenchymal Stem Cells Promote Facial Nerve Regeneration in Rats. Mol Neurobiol 55, 6965–6983 (2018). https://doi.org/10.1007/s12035-018-0913-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0913-3