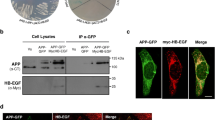
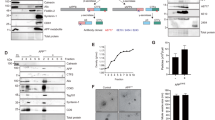
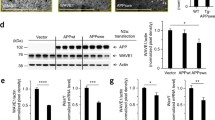
Abstract
The amyloid precursor protein (APP) undergoes extensive metabolism, and its transport and proteolytic processing can be modulated by its ability to form a homodimer. We have investigated the functional consequences of stabilised APP dimer expression in cells by studying the engineered dimerisation of the APPL17C (residue 17 in Aβ sequence) construct, which is associated with a 30% increase in APP dimer expression, on APP’s neurite outgrowth promoting activity. Overexpression of APPL17C in SH-SY5Y cells decreased neurite outgrowth upon retinoic acid differentiation as compared to overexpressing APPWT cells. The APPL17C phenotype was rescued by replacing the APPL17C media with conditioned media from APPWT cells, indicating that the APPL17C mutant is impairing the secretion of a neuritogenic promoting factor. APPL17C had altered transport and was localised in the endoplasmic reticulum. Defining the molecular basis of the APPL17C phenotype showed that RhoA GTPase activity, a negative regulator of neurite outgrowth, was increased in APPL17C cells. RhoA activity was decreased after APPWT conditioned media rescue. Moreover, treatment with the RhoA inhibitor, Y27632, restored a wild-type morphology to the APPL17C cells. Small RNAseq analysis of APPL17C and APPWT cells identified several differentially expressed miRNAs relating to neurite outgrowth. Of these, miR-34a showed the greatest decrease in expression. Lentiviral-mediated overexpression of miR-34a rescued neurite outgrowth in APPL17C cells to APPWT levels and changed RhoA activation. This study has identified a novel link between APP dimerisation and its neuritogenic activity which is mediated by miR-34a expression.










Similar content being viewed by others
References
Muller UC, Zheng H (2012) Physiological functions of APP family proteins. Cold Spring Harb Perspect Med 2(2):a006288. https://doi.org/10.1101/cshperspect.a006288
Muller UC, Deller T, Korte M (2017) Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci 18(5):281–298. https://doi.org/10.1038/nrn.2017.29
Sosa LJ, Caceres A, Dupraz S, Oksdath M, Quiroga S, Lorenzo A (2017) The physiological role of the amyloid precursor protein as an adhesion molecule in the developing nervous system. J Neurochem 143(1):11–29. https://doi.org/10.1111/jnc.14122
Thordardottir S, Kinhult Stahlbom A, Almkvist O, Thonberg H, Eriksdotter M, Zetterberg H, Blennow K, Graff C (2017) The effects of different familial Alzheimer’s disease mutations on APP processing in vivo. Alzheimers Res Ther 9(1):9. https://doi.org/10.1186/s13195-017-0234-1
Gerber H, Wu F, Dimitrov M, Garcia Osuna GM, Fraering PC (2017) Zinc and copper differentially modulate amyloid precursor protein processing by gamma-secretase and amyloid-beta peptide production. J Biol Chem 292(9):3751–3767. https://doi.org/10.1074/jbc.M116.754101
Klaver D, Hung AC, Gasperini R, Foa L, Aguilar MI, Small DH (2010) Effect of heparin on APP metabolism and Abeta production in cortical neurons. Neurodegener Dis 7(1–3):187–189. https://doi.org/10.1159/000295661
Deyts C, Thinakaran G, Parent AT (2016) APP receptor? To be or not to be. Trends Pharmacol Sci 37(5):390–411. https://doi.org/10.1016/j.tips.2016.01.005
Soba P, Eggert S, Wagner K, Zentgraf H, Siehl K, Kreger S, Lower A, Langer A et al (2005) Homo- and heterodimerization of APP family members promotes intercellular adhesion. EMBO J 24(20):3624–3634. https://doi.org/10.1038/sj.emboj.7600824
Munter LM, Voigt P, Harmeier A, Kaden D, Gottschalk KE, Weise C, Pipkorn R, Schaefer M et al (2007) GxxxG motifs within the amyloid precursor protein transmembrane sequence are critical for the etiology of Abeta42. EMBO J 26(6):1702–1712. https://doi.org/10.1038/sj.emboj.7601616
Kienlen-Campard P, Tasiaux B, Van Hees J, Li M, Huysseune S, Sato T, Fei JZ, Aimoto S et al (2008) Amyloidogenic processing but not amyloid precursor protein (APP) intracellular C-terminal domain production requires a precisely oriented APP dimer assembled by transmembrane GXXXG motifs. J Biol Chem 283(12):7733–7744. https://doi.org/10.1074/jbc.M707142200
Qiu WQ, Ferreira A, Miller C, Koo EH, Selkoe DJ (1995) Cell-surface beta-amyloid precursor protein stimulates neurite outgrowth of hippocampal neurons in an isoform-dependent manner. J Neurosci 15(3 Pt 2):2157–2167
Alvarez J, Moreno RD, Llanos O, Inestrosa NC, Brandan E, Colby T, Esch FS (1992) Axonal sprouting induced in the sciatic nerve by the amyloid precursor protein (APP) and other antiproteases. Neurosci Lett 144(1–2):130–134. https://doi.org/10.1016/0304-3940(92)90733-N
Perez RG, Zheng H, Van der Ploeg LH, Koo EH (1997) The beta-amyloid precursor protein of Alzheimer’s disease enhances neuron viability and modulates neuronal polarity. J Neurosci 17(24):9407–9414
Williamson TG, Mok SS, Henry A, Cappai R, Lander AD, Nurcombe V, Beyreuther K, Masters CL et al (1996) Secreted glypican binds to the amyloid precursor protein of Alzheimer’s disease (APP) and inhibits APP-induced neurite outgrowth. J Biol Chem 271(49):31215–31221. https://doi.org/10.1074/jbc.271.49.31215
Allinquant B, Hantraye P, Mailleux P, Moya K, Bouillot C, Prochiantz A (1995) Downregulation of amyloid precursor protein inhibits neurite outgrowth in vitro. J Cell Biol 128(5):919–927. https://doi.org/10.1083/jcb.128.5.919
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, Liuyang ZY, Roisman L et al (2017) Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry 22(11):1520–1530. https://doi.org/10.1038/mp.2017.171
Savage C, Hamelin M, Culotti JG, Coulson A, Albertson DG, Chalfie M (1989) Mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes Dev 3(6):870–881. https://doi.org/10.1101/gad.3.6.870
Chan CC, Khodarahmi K, Liu J, Sutherland D, Oschipok LW, Steeves JD, Tetzlaff W (2005) Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol 196(2):352–364. https://doi.org/10.1016/j.expneurol.2005.08.011
Nakayama AY, Harms MB, Luo L (2000) Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci 20(14):5329–5338
Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S (2000) Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 57(5):976–983
Makeyev EV, Zhang J, Carrasco MA, Maniatis T (2007) The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27(3):435–448. https://doi.org/10.1016/j.molcel.2007.07.015
Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439(7074):283–289. https://doi.org/10.1038/nature04367
Corrigan F, Pham CL, Vink R, Blumbergs PC, Masters CL, van den Heuvel C, Cappai R (2011) The neuroprotective domains of the amyloid precursor protein, in traumatic brain injury, are located in the two growth factor domains. Brain Res 1378:137–143. https://doi.org/10.1016/j.brainres.2010.12.077
Dinet V, An N, Ciccotosto GD, Bruban J, Maoui A, Bellingham SA, Hill AF, Andersen OM et al (2011) APP involvement in retinogenesis of mice. Acta Neuropathol 121(3):351–363. https://doi.org/10.1007/s00401-010-0762-2
Needham BE, Wlodek ME, Ciccotosto GD, Fam BC, Masters CL, Proietto J, Andrikopoulos S, Cappai R (2008) Identification of the Alzheimer’s disease amyloid precursor protein (APP) and its homologue APLP2 as essential modulators of glucose and insulin homeostasis and growth. J Pathol 215(2):155–163. https://doi.org/10.1002/path.2343
Needham BE, Ciccotosto GD, Cappai R (2014) Combined deletions of amyloid precursor protein and amyloid precursor-like protein 2 reveal different effects on mouse brain metal homeostasis. Metallomics 6(3):598–603. https://doi.org/10.1039/c3mt00358b
Masters CL, Selkoe DJ (2012) Biochemistry of amyloid beta-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med 2(6):a006262. https://doi.org/10.1101/cshperspect.a006262
Munter LM, Botev A, Richter L, Hildebrand PW, Althoff V, Weise C, Kaden D, Multhaup G (2010) Aberrant amyloid precursor protein (APP) processing in hereditary forms of Alzheimer disease caused by APP familial Alzheimer disease mutations can be rescued by mutations in the APP GxxxG motif. J Biol Chem 285(28):21636–21643. https://doi.org/10.1074/jbc.M109.088005
Noda Y, Asada M, Kubota M, Maesako M, Watanabe K, Uemura M, Kihara T, Shimohama S et al (2013) Copper enhances APP dimerization and promotes Abeta production. Neurosci Lett 547:10–15. https://doi.org/10.1016/j.neulet.2013.04.057
Isbert S, Wagner K, Eggert S, Schweitzer A, Multhaup G, Weggen S, Kins S, Pietrzik CU (2012) APP dimer formation is initiated in the endoplasmic reticulum and differs between APP isoforms. Cell Mol Life Sci 69(8):1353–1375. https://doi.org/10.1007/s00018-011-0882-4
Khalifa NB, Van Hees J, Tasiaux B, Huysseune S, Smith SO, Constantinescu SN, Octave JN, Kienlen-Campard P (2010) What is the role of amyloid precursor protein dimerization? Cell Adhes Migr 4(2):268–272
Baumkotter F, Schmidt N, Vargas C, Schilling S, Weber R, Wagner K, Fiedler S, Klug W et al (2014) Amyloid precursor protein dimerization and synaptogenic function depend on copper binding to the growth factor-like domain. J Neurosci 34(33):11159–11172. https://doi.org/10.1523/jneurosci.0180-14.2014
da Rocha JF, da Cruz e Silva OA, Vieira SI (2015) Analysis of the amyloid precursor protein role in neuritogenesis reveals a biphasic SH-SY5Y neuronal cell differentiation model. J Neurochem 134(2):288–301. https://doi.org/10.1111/jnc.13133
Adlerz L, Beckman M, Holback S, Tehranian R, Cortes Toro V, Iverfeldt K (2003) Accumulation of the amyloid precursor-like protein APLP2 and reduction of APLP1 in retinoic acid-differentiated human neuroblastoma cells upon curcumin-induced neurite retraction. Brain Res Mol Brain Res 119(1):62–72. https://doi.org/10.1016/j.molbrainres.2003.08.014
Holback S, Adlerz L, Iverfeldt K (2005) Increased processing of APLP2 and APP with concomitant formation of APP intracellular domains in BDNF and retinoic acid-differentiated human neuroblastoma cells. J Neurochem 95(4):1059–1068. https://doi.org/10.1111/j.1471-4159.2005.03440.x
Vella LJ, Cappai R (2012) Identification of a novel amyloid precursor protein processing pathway that generates secreted N-terminal fragments. FASEB J 26(7):2930–2940. https://doi.org/10.1096/fj.11-200295
LeBlanc AC, Goodyer CG (1999) Role of endoplasmic reticulum, endosomal-lysosomal compartments, and microtubules in amyloid precursor protein metabolism of human neurons. J Neurochem 72(5):1832–1842. https://doi.org/10.1046/j.1471-4159.1999.0721832.x
Greenfield JP, Tsai J, Gouras GK, Hai B, Thinakaran G, Checler F, Sisodia SS, Greengard P et al (1999) Endoplasmic reticulum and trans-Golgi network generate distinct populations of Alzheimer β-amyloid peptides. Proc Natl Acad Sci U S A 96(2):742–747. https://doi.org/10.1073/pnas.96.2.742
Ben Khalifa N, Tyteca D, Marinangeli C, Depuydt M, Collet JF, Courtoy PJ, Renauld JC, Constantinescu S et al (2012) Structural features of the KPI domain control APP dimerization, trafficking, and processing. FASEB J 26(2):855–867. https://doi.org/10.1096/fj.11-190207
Chen C, Wirth A, Ponimaskin E (2012) Cdc42: an important regulator of neuronal morphology. Int J Biochem Cell Biol 44(3):447–451. https://doi.org/10.1016/j.biocel.2011.11.022
Fujita Y, Yamashita T (2014) Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci 8:338. https://doi.org/10.3389/fnins.2014.00338
Kranenburg O, Poland M, Gebbink M, Oomen L, Moolenaar WH (1997) Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. J Cell Sci 110 (Pt 19) (19):2417–2427
Hasebe N, Fujita Y, Ueno M, Yoshimura K, Fujino Y, Yamashita T (2013) Soluble beta-amyloid precursor protein alpha binds to p75 neurotrophin receptor to promote neurite outgrowth. PLoS One 8(12):e82321. https://doi.org/10.1371/journal.pone.0082321
Yamashita T, Tucker KL, Barde YA (1999) Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron 24(3):585–593. https://doi.org/10.1016/S0896-6273(00)81114-9
Zhang YW, Chen Y, Liu Y, Zhao Y, Liao FF, Xu H (2013) APP regulates NGF receptor trafficking and NGF-mediated neuronal differentiation and survival. PLoS One 8(11):e80571. https://doi.org/10.1371/journal.pone.0080571
Chasseigneaux S, Dinc L, Rose C, Chabret C, Coulpier F, Topilko P, Mauger G, Allinquant B (2011) Secreted amyloid precursor protein beta and secreted amyloid precursor protein alpha induce axon outgrowth in vitro through Egr1 signaling pathway. PLoS One 6(1):e16301. https://doi.org/10.1371/journal.pone.0016301
Gu X, Meng S, Liu S, Jia C, Fang Y, Li S, Fu C, Song Q et al (2014) miR-124 represses ROCK1 expression to promote neurite elongation through activation of the PI3K/Akt signal pathway. J Mol Neurosci 52(1):156–165. https://doi.org/10.1007/s12031-013-0190-6
Fang W, Gao G, Zhao H, Xia Y, Guo X, Li N, Li Y, Yang Y et al (2015) Role of the Akt/GSK-3beta/CRMP-2 pathway in axon degeneration of dopaminergic neurons resulting from MPP+ toxicity. Brain Res 1602:9–19. https://doi.org/10.1016/j.brainres.2014.08.030
Carrel D, Firestein BL (2009) MicroRNA-mediated regulation of synaptic palmitoylation: shrinking fat spines. Nat Cell Biol 11(6):681–682. https://doi.org/10.1038/ncb0609-681
Schratt G (2009) microRNAs at the synapse. Nat Rev Neurosci 10(12):842–849. https://doi.org/10.1038/nrn2763
Nampoothiri SS, Rajanikant GK (2017) Decoding the ubiquitous role of microRNAs in neurogenesis. Mol Neurobiol 54(3):2003–2011. https://doi.org/10.1007/s12035-016-9797-2
Jang J, Lee S, Oh HJ, Choi Y, Choi JH, Hwang DW, Lee DS (2016) Fluorescence imaging of in vivo miR-124a-induced neurogenesis of neuronal progenitor cells using neuron-specific reporters. EJNMMI Res 6(1):38. https://doi.org/10.1186/s13550-016-0190-y
Lin L, Gu X, Liu S, Wang X (2014) miR-124a promotes neurite outgrowth by inhibiting iASPP expression. Nan Fang Yi Ke Da Xue Xue Bao 34(1):31–35. https://doi.org/10.3969/j.issn.1673-4254.2014.01.07
Agostini M, Tucci P, Steinert JR, Shalom-Feuerstein R, Rouleau M, Aberdam D, Forsythe ID, Young KW et al (2011) microRNA-34a regulates neurite outgrowth, spinal morphology, and function. Proc Natl Acad Sci U S A 108(52):21099–21104. https://doi.org/10.1073/pnas.1112063108
Funding
This work was supported from funding from the Australia National Health and Medical Research Council (R.C., A.F.H.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Luu, L., Ciccotosto, G.D., Vella, L.J. et al. Amyloid Precursor Protein Dimerisation Reduces Neurite Outgrowth. Mol Neurobiol 56, 13–28 (2019). https://doi.org/10.1007/s12035-018-1070-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1070-4