Abstract
The classical amyloid cascade model for Alzheimer’s disease (AD) has been challenged by several findings. Here, an alternative molecular neurobiological model is proposed. It is shown that the presence of the APOE ε4 allele, altered miRNA expression and epigenetic dysregulation in the promoter region and exon 1 of TREM2, as well as ANK1 hypermethylation and altered levels of histone post-translational methylation leading to increased transcription of TNFA, could variously explain increased levels of peripheral and central inflammation found in AD. In particular, as a result of increased activity of triggering receptor expressed on myeloid cells 2 (TREM-2), the presence of the apolipoprotein E4 (ApoE4) isoform, and changes in ANK1 expression, with subsequent changes in miR-486 leading to altered levels of protein kinase B (Akt), mechanistic (previously mammalian) target of rapamycin (mTOR) and signal transducer and activator of transcription 3 (STAT3), all of which play major roles in microglial activation, proliferation and survival, there is activation of microglia, leading to the subsequent (further) production of cytokines, chemokines, nitric oxide, prostaglandins, reactive oxygen species, inducible nitric oxide synthase and cyclooxygenase-2, and other mediators of inflammation and neurotoxicity. These changes are associated with the development of amyloid and tau pathology, mitochondrial dysfunction (including impaired activity of the electron transport chain, depleted basal mitochondrial potential and oxidative damage to key tricarboxylic acid enzymes), synaptic dysfunction, altered glycogen synthase kinase-3 (GSK-3) activity, mTOR activation, impairment of autophagy, compromised ubiquitin-proteasome system, iron dyshomeostasis, changes in APP translation, amyloid plaque formation, tau hyperphosphorylation and neurofibrillary tangle formation.
Similar content being viewed by others
Introduction
Alzheimer’s disease (AD) is a progressive, clinically heterogeneous, age-sensitive neurodegenerative disease, characterised by often escalating impairments of memory and other cognitive functions together with associated changes in personality and behaviour [1,2,3]. Amyloid plaques and neurofibrillary tangles (NFTs) are invariant pathological hallmarks seen in the brains of people suffering from of AD [4]. These abnormalities are held to result from the accumulation of small peptides known as amyloid beta (Aβ) in central nervous system (CNS) tissues, and from gross changes in cytoskeletal organisation stemming from the hyperphosphorylation of the microtubule-associated protein tau (ptau) in neurones [5]. According to the classical ‘amyloid cascade’ model of disease causation, Aβ is overproduced following the disruption of homeostatic mechanisms which normally regulate the proteolytic cleavage of the amyloid precursor protein (APP). In this model, age-related genetic and environmental factors conspire to induce a metabolic shift favouring the amyloidogenic processing of APP but inhibiting the physiological, secretory pathway [6,7,8]. These processes are represented in Figs. 1 and 2 and are well documented and hence will not be the main focus of this paper.
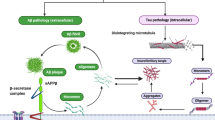
The amyloid hypothesis. According to the current ‘amyloid cascade’ model of disease causation, Aβ overproduction stems from the disruption of homeostatic mechanisms that regulate the proteolytic cleavage of APP under physiological conditions. This model proposes that age-related, genetic, epigenetic and environmental factors collude to provoke a metabolic shift favouring the processing of APP by BACE1 and the intramembranous γ-secretase complex composed in part by presenilin-1 or presenilin-2, while simultaneously inhibiting the physiological, secretory pathway via α-secretase, which releases soluble APPα which precludes generation of Aβ. The net result is to enhance the production of the putatively neurotoxic Aβ42 monomer at the expense of the putatively neuroprotective Aβ40. The current version of the amyloid hypothesis claims that Aβ42 accumulation into soluble oligomers is the primary driver of neuropathology, although the data allow for an independent or synergistic role for insoluble fibrils
Physiological and pathological APP processing. APP is processed via two mutually exclusive pathways involving cleavage by β-secretase and α-secretase. Cleavage by the latter enzyme intersects the β-amyloid region, which eliminates the possibility of Aβ production and produces membrane-bound C83 protein and sAPPα which enters the cytosol. Subsequent processing of C83 by γ-secretase generates p3 and Aβ together with the amino-terminal APP intracellular domain (AICD). APP cleavage by β-secretase results in the production sAPPβ and C99. Further processing of C99 leads to the production of the AICD fragment and Aβ which forms oligomers and ultimately fibrils
The amyloid hypothesis has been under challenge in recent years as a result of several findings. One is the failure of human trials using therapies targeting the amyloid cascade; another is evidence obtained from positron emission tomography neuroimaging demonstrating increased amyloid accumulation in cognitively intact individuals and an absence of correlation between amyloid load and disease severity in AD patients and in cognitively normal individuals [9, 10].
Hence, while the hypothesis proposing a causative role for Aβ oligomers and ptau as the main, or at least initial, instigator of pathology in AD at least in advanced disease probably holds primacy, there is a growing consensus that the maintenance if not the origin of AD pathology is multifactorial, likely with a high degree of inter-patient heterogeneity [11,12,13,14]. This is unsurprising as there is now an extensive body of evidence showing that there are many potential drivers of pathology in the brains of patients diagnosed with AD or mild cognitive impairment (MCI) which are evident in patients with MCI long before the development of amyloid plaques or neurofibrillary tangles (reviewed by [15]). Chronic nitrosative and oxidative stress and significantly depleted levels of reduced glutathione are invariant but non-specific findings, as is the existence of impaired mitochondrial function along many dimensions [16,17,18,19].
The presence of activated and dysfunctional microglia and reactive astrogliosis would also seem to be an invariant finding in vivo both in AD and MCI [20,21,22]. Other commonly reported abnormalities include compromised autophagy and lysosomal clearance accompanied by elevated activity of both glycogen synthase kinase-3 (GSK-3) and mechanistic (previously mammalian) target of rapamycin (mTOR), coupled with a defective ubiquitin-proteasome system (UPS) [12, 23,24,25,26,27]. Several authors have also reported abnormalities in the activity of several kinases and phosphatases, most notably mitogen-activated protein kinases (MAPKs) and protein phosphatase 2A (PP2A or PP2), and transition metal dyshomeostasis, which could all arguably play a role, either as primary or secondary drivers of disease activity [11,12,13, 16, 28,29,30].
There is a growing consensus that iron dyshomeostasis plays a pivotal pathological role in the illness, with increased levels of iron proposed as the primary driver of neurodegeneration by many research teams [31,32,33,34,35]. Peripheral immune abnormalities and inflammation are also being increasingly advocated as major, albeit again non-specific, drivers of symptoms [36, 37]. Abnormalities in the composition of the microbiota, and translocation of bacterial antigens into the systemic circulation and the brain, have also become areas of intense research across the neurosciences [38, 39].
Impaired cerebral glucose metabolism is also invariantly reported in AD patients and its occurrence precedes symptoms sometimes for years or even decades [40]. Moreover, the progressive increase in the levels and topography of glucose hypometabolism correlates with an increase in symptom severity and synaptic dysfunction review [40]. In this context, the presence of insulin resistance in AD is unsurprising (reviewed by [41]). This is also concordant with type 2 diabetes mellitus being a risk factor for AD. These observations are of interest as they are common to both disorders and could be explained by the presence of chronic inflammation, oxidative stress and mitochondrial dysfunction in the periphery and brain [42,43,44,45]. Chronic inflammation and oxidative stress are also acknowledged causes of GSK-3 and mTOR upregulation and could also account for Aβ upregulation (reviewed by [15]). These observations rather invite the question as to whether increased peripheral inflammation and oxidative stress could be a major driver of the abnormalities repeatedly reported in AD patients. However, it should be noted that these abnormalities have also been repeatedly reported in cognitively intact elderly people as well as in diverse medical and neuropsychiatric disorders [46,47,48,49,50,51,52,53,54]; hence, there must be other genetic and/or epigenetic factors involved.
Genome-wide association studies (GWASs) have revealed that approximately 40% of AD patients carry the apolipoprotein E (APOE) ε4 allele and that APOE ε4-positive, but cognitively intact, individuals over 50 years of age are significantly more likely to have brain amyloid deposits than individuals free of that polymorphism [55]; reviewed in [56]. There is also evidence that, compared with age- and sex-matched controls, AD patients carrying both the APOE ε4 allele and the H63D polymorphism of the hemochromatosis protein-related class I-like major histocompatibility gene HFE are significantly more susceptible to earlier development of AD than those carrying only one of these mutations [57]; reviewed by [58]. More recently, researchers have detected the rs75932628 single-nucleotide polymorphism (SNP) within the triggering receptor expressed on myeloid cells 2 (TREM2) gene, leading to an R47H substitution, which increases the risk of developing AD in carriers by virtually the same magnitude as the presence of one APOE ε4 allele [59]; reviewed by [60]. However, while genetics clearly plays a role in AD susceptibility, the vast bulk of cases does not show strong genetic underpinnings [61, 62]. Moreover, although common sequence variants in several genes display robust associations with AD susceptibility, evidenced by individual studies and subsequent meta-analyses, collectively, these SNPs only account for approximately a third of attributable risk and the mechanisms underpinning these associations remain undelineated [63]; reviewed by [64].
Recent epigenetic-wide association studies (EWASs) have revealed that AD may be associated with decreased histone acetylation, increased histone phosphorylation (probably including neuronal histone hyperphosphorylation) and DNA hypermethylation with likely increased CpG methylation [62]. Moreover, several research teams have independently reported strong associations between the epigenetic dysregulation of a range of genes and the development of AD in entirely asymptomatic patients (reviewed in [61]). Changes in the methylation status of ANK-1 which encodes ankyrin repeat domain-containing protein 1, which plays a role in linking integral membrane proteins to the spectrin-actin cytoskeleton, display a particularly strong association with AD development and the burden of neuropathology [65, 66].
Moreover, recent data implicating allele-specific changes to the methylation status of the CpG islands (CGI) responsible for the transcription of APOE and downstream genes in AD patients may offer a better understanding of the mechanisms underpinning the increased risk of developing the disease in carriers of the APOE ε4 allele [67, 68]. This may also be the case for TREM2, as a recent meta-analysis concluded that increased methylation of the TREM2 promoter region appears to be an invariant feature in the brains of AD patients independently of age and sex [64]. Moreover, this increase in methylation correlates with a higher level of TREM-2 (triggering receptor expressed on myeloid cells 2) activity in the brains of AD patients compared with healthy age- and sex-matched controls [69]. It is also noteworthy that, when viewed as a whole, the results of EWASs indicate that epigenetic abnormalities in tandem with increased levels of inflammation greatly exacerbate the risk of developing AD [61, 65, 66]. In the light of the above, this paper focuses on three questions. First, can genetic and epigenetic factors explain increased levels of peripheral and central inflammation and oxidative stress in AD? Second, could this increased oxidative stress and inflammation originate in the periphery? Third, can the initial development of elevated peripheral and central inflammation and oxidative stress in the context of genetic and epigenetic abnormalities explain the development of AD?
Evidence of Peripheral Inflammation and Immune Abnormalities in AD
Evidence of Peripheral Inflammation in AD
Two large meta-analyses have confirmed the presence of elevated pro-inflammatory cytokines (PICs) and other inflammatory molecules in the serum and whole blood of AD patients. In the first of these studies, Swardfager and fellow workers analysed the results of 40 studies and reported a higher inflammatory status, evidenced by elevated levels of interleukin 6 (IL-6), IL-12, tumour necrosis factor-alpha (TNF-α), IL-1β and IL-18, compared with age- and sex-matched healthy controls [37]. These results have been confirmed in a more recent meta-analysis of 175 studies involving 13,344 AD patients and 12,912 healthy controls conducted by Lai and others [70]. These authors reported elevated levels of TNF-α converting enzyme, soluble TNF receptors 1 and 2, IL-6, IL-8, C-X-C motif chemokine-10, IL-2, α1-antichymotrypsin, high-sensitivity C-reactive protein and homocysteine. This meta-analysis also revealed decreased levels of leptin and IL-1 receptor antagonist in AD patients and it is noteworthy that these authors concluded that IL-6 levels were inversely correlated with cognitive scores as ascertained by the Mini-Mental State Examination (MMSE) [70]. The last finding is unsurprising as there is a large body of evidence confirming that inflammatory signals can have a severe adverse effect on brain function, and is consistent with the work of several research teams which have reported that PIC levels in AD patients are positively associated with cognitive decline, increased frequency and severity of neuropsychiatric symptoms, disease severity and overall disease progression [71,72,73,74,75,76].
It is also worth noting that the combination of PIC levels and brain magnetic resonance imaging (MRI) measures is more predictive of the transition from mild cognitive impairment (MCI) to AD than APOE genotype status alone [77, 78]. The weight of data indicates that concentrations of TNF-α in particular appear to have a clear effect on disease progression and/or severity. For example, Holmes and fellow workers reported that a twofold increase in serum TNF-α levels over a 6-month period, indexing successive inflammatory insults, was associated with a twofold rate of cognitive decline over the same period [72]. Furthermore, high baseline levels of the cytokine were associated with a fourfold decline in cognitive function while patients with population-normal levels of TNF-α experienced no cognitive decline over the course of the study [72]. These results were broadly replicated in a later study conducted by the same research team, who reported that TNF-α and IL-6 levels correlated with an increased frequency of neuropsychiatric symptoms characteristic of pathogen-induced sickness behaviour [75]. Finally, a more recent study established a relationship between elevated levels of TNF-α, IL-6 and interferon gamma (IFNγ), produced by abnormally activated T cells, and disease severity [76].
Evidence of Peripheral Immune Abnormalities in AD
Several research teams have reported abnormalities in CD4 and CD8 T cell activation, differentiation, trafficking and receptor expression in patients with MCI and AD compared with age- and sex-matched controls, although the results reported by different research teams vary [36, 79]; reviewed in [80]. The weight of evidence indicates that CD4 T cells are activated and highly differentiated in AD patients as indicated by a reduction in naïve and central memory CD4+ T cells, an increase in Th17 T cells and a reduction in regulatory T cells (Tregs) [34, 81, 82]. In addition, the pattern of receptor distribution on the surface of CD4 T cells may also differ between AD patients and age- and sex-matched controls, with an increased number of CD4+ CD28− cells being reported [34]. There is some evidence that the pattern of CD4 T cell activity may be different in patients with MCI compared with AD in whom Treg activity appears to be increased possibly in an attempt to combat increasing inflammation [35].
The data regarding various aspects of CD8 T cell abnormalities in AD patients are mixed and often conflicting with increased numbers and activity, decreased numbers and activity and no changes compared with age- and sex-matched controls all being reported [34, 36, 83]. However, several authors have suggested that these inconsistencies could potentially be explained by the different methods used and differences in compartments sampled [80].
The pathogenic significance, if any, of these T cell abnormalities is still a matter of debate but there is a growing body of evidence that the entry of activated CD4 and CD8 T cells into the CNS and dysfunctional ‘cross talk’ between the CNS and the peripheral immune system make a significant contribution to the genesis and/or exacerbation of pathology in at least some patients with AD [84, 85]. In this context, it is noteworthy that several research teams have reported the presence of CD4 and CD8 T cells in the brains of AD patients post mortem (reviewed by [86]) and a recent study has reported a significant correlation between the extent of CD8 T cell activation and parahippocampal microstructural tissue damage in AD patients [83]. Moreover, this last team of authors reported that levels of activated HLA-DR-positive CD4+ and CD8+ T cells were significantly increased in the peripheral blood of AD and MCI patients compared with age- and sex-matched controls, but not in patients with a range of non-AD dementias [83]. This finding is consistent with that of other published research which indicates that the pattern of T cell abnormalities seen in AD may well be specific to the disease [87, 88].
Potential Origins of Peripheral Inflammation and Immune Activation in AD
The Presence of Serum Aβ Autoantibodies
The origin of the chronic peripheral activation and activated but dysregulated immune system seen in AD patients has not been delineated, but the presence of autoantibodies directed at Aβ in the serum of AD patients, possibly as a result of efflux from the brain, should be considered as certain classes of antibody are well-documented inflammatory mediators [36]. The evidence regarding the existence of increased levels of these antibodies in AD patients compared with age- and sex-matched controls is unconvincing, however, with elevated levels, reduced levels and no significant differences being reported (reviewed by [89]). It is also worthy of note that the levels of B cells producing autoantibodies against Aβ appear to be the same in AD patients and healthy controls [90, 91]. Moreover, thus far, all available evidence demonstrates that these autoantibodies (both IgM and IgG) are catalytic in nature, meaning that they rarely form stable complexes and are not recognised sources of inflammation [92, 93]. The lack of association between serum Aβ autoantibody levels and Aβ levels in the brain reported by Xu and others is also relevant as this finding casts doubt on the origin of serum Aβ [91]. The lack of T cell responses to Aβ in AD patients reported by Baril and colleagues is also pertinent; this finding renders the hypothesis that antibodies to Aβ in the serum of AD patients are the cause of T cell activation and differentiation patterns in such patients improbable, although it cannot be ruled out [94].
Dysbiosis and Translocation of Commensal Lipopolysaccharide
Another possible cause could stem from disturbances in the composition of the microbiota and translocation of commensal LPS into the peripheral circulation, which have both been recently reported in AD, although this again is a very non-specific finding [38, 39]. The inflammatory consequences of this latter phenomenon, achieved via activation of toll-like receptors on the surfaces of macrophages and dendritic cells and the subsequent production of PICs, are well documented and hence bacterial translocation as a consequence of increased intestinal permeability could go some way to explaining chronic systemic inflammation in AD (reviewed by [95, 96]).
Increased levels of translocated LPS can also have profound effects on T cell activation, differentiation and trafficking, and thus could potentially explain at least some of the peripheral T cell abnormalities seen in AD patients. For example, LPS activation of antigen-presentation cells (APCs) via TRIF (TIR (toll/IL-1 receptor) domain-containing adaptor-inducing IFNβ)- and MyD88 (myeloid differentiation primary response 88)-dependent signalling pathways initiates CD4 T helper cell clonal expansion and differentiation [97]. The effect of LPS exposure on CD4 T cell differentiation appears to be tissue dependent as evidenced by reports of Th1 cell differentiation being induced by the presence of LPS in lymphoid tissue and Th17 cell differentiation being the result of naïve CD4 T cell exposure to LPS in the intestinal lamina propria [97]. LPS also affects T cell differentiation indirectly by stimulating B cells via a mechanism involving toll-like receptor-4 (TLR-4) and B cell-activating factor belonging to TNF superfamily (BAFF) activation, which results in naïve CD4 T cell differentiation towards a Th2 or Treg lineage depending on localised levels of that commensal antigen [98]; reviewed in [99]. Finally, it has been suggested that activation of TLR-4 receptors on CD4 T cells by LPS may predispose to the development of autoimmunity as such activation appears to increase the proliferation and inflammatory status and survival of Th1 and Th17 cells [100].
Translocated LPS would also appear to exert a range of effects on CD8+ T cell activation, differentiation, survival and trafficking. For example, Cui and fellow workers reported increased proliferation and survival of memory CD8+ T lymphocytes in an environment of high LPS, while McAleer and others reported increased CD8+ T cell trafficking into non-lymphoid tissue under similar conditions [101, 102]. The surface TLR-4 receptors are directly sensitive to the presence of LPS and thus evidence demonstrating their activation in an environment of high LPS, as characterised by elevated levels of CD25 and CD69 receptors, in the absence of APC activation, is unsurprising [103]. This interaction would appear to be of considerable pathogenic importance in vivo and is now considered to be a major driver of tissue damage in rheumatoid arthritis [104], which is of interest given the data implicating increased CD8+ T cell activation levels and numbers as a driver of tissue damage in AD as described above.
There is evidence to suggest that LPS also induces synthesis of IFNγ by natural killer (NK) cells via a mechanism which does not appear to involve TLR-4 activation on APCs [105], and there are replicated data indicating that the presence of this antigen stimulates the proliferation of CD56+ CD3− NK cells, which appear to play a role in the pathogenesis of AD [106, 107]; reviewed in [108].
APOE plays a regulatory role in inflammatory signalling in APCs and there is some evidence to suggest that the APOE ε4 allele is associated with higher levels of PIC production by LPS-activated macrophages via upregulation of NF-κB transcription resulting in increased levels of TNF-α and IL-1 with a concomitant reduction in IL-10, which is of interest given the probable role of translocated LPS in the aetiology of peripheral inflammation in AD discussed above [109, 110].
Cash and colleagues studied mice in which the endogenous apoe gene was replaced, at the same locus, by either the human APOE4 or APOE3 gene; compared with the APOE3 mice, the APOE4 ones showed defective macrophagic efferocytosis, which is a process involving the phagocytosis and immunologically silent clearance of dying and dead cells [111]. This may have significant pathological consequences given considerable data indicating that tissue inflammation may result from the failure of this mechanism; impaired efferocytosis is being increasingly implicated in the pathogenesis of autoinflammatory and autoimmune diseases [112].
The presence of dysbiosis in AD patients, which seems to involve increased Bacteroidetes, decreased Firmicutes and Actinobacteria (including decreased Bifidobacterium and Adlercreutzia genera) phyla compared with age- and sex-matched controls [38], may also contribute to the Th17/Treg imbalance reported in AD, as described above. Several research teams have independently reported that the composition of the microbiota plays a key role in determining the trajectory of activated naïve CD4 T cell differentiation along the Th17 or Treg pathways [113, 114]. It should be noted that there are few Th17 cells in lymph nodes and the vast bulk of this T cell population resides in the intestinal lamina propria and can home in to the blood and other peripheral tissues following activation, and therefore can be a source of the systemically elevated T cells of this type reported in AD [95, 115]. Intriguingly, there is also accumulating evidence suggesting that gut microbiota profiles influence the DNA methylation patterns of T cells and other cellular inhabitants in the blood, thereby determining, at least to some extent, the inflammatory status of an individual [116].
This is a complex area and readers interested in pursuing this matter are invited to consult an excellent and comprehensive review conducted by Ye and fellow workers [117]. The class of apolipoprotein E (ApoE) proteins plays a major role in regulating intestinal immune system homeostasis, colonic inflammation and composition of the microbiota, and therefore it is tempting to speculate that APOE ε4 allele status could be associated with pathological changes in all these parameters; however, it must be stressed that there is no evidence regarding this area in AD or indeed any other disease [118].
Epigenetic Changes in Peripheral Mononuclear Blood Cells
There is evidence of epigenetic dysregulation in the T cells and macrophages of AD patients compared with age- and sex-matched controls, with increased expression of microRNA-155 (miR-155) being reported in T cells and differential DNA methylation changes being observed in CD14+ macrophages [119, 120]. These findings could also potentially indicate a source of elevated peripheral inflammation in AD as miR-155 is NF-κB sensitive and also acts to increase the transcription of NF-κB, which in turn allows for increasing levels of inflammation and PIC production by activated T cells in a positive feedback loop [121, 122]. While the origin of increased expression of miR-155 in AD is not known, one potential cause could be translocated LPS which is documented to increase the expression of this molecule in human peripheral mononuclear blood cells (PMBCs) most notably macrophages [123].
There is also an accumulating body of evidence implicating epigenetic dysregulation, most notably increased DNA methylation and altered miRNA expression, with an elevated inflammatory status of macrophages [124, 125]. For example, Wang and colleagues reported that obesity-induced hypermethylation of DNA in the promoter region of peroxisome proliferator-activated receptor γ1 (PPARγ1) exacerbated the inflammatory status of macrophages and provoked a polarisation towards the M1 phenotype [124]. These findings were also reported by Yang and fellow workers in an earlier study [126]. miRNA profiles and levels also regulate the inflammatory status and polarisation of macrophages via several different mechanisms including NF-κB transcription and cellular location [127, 128].
APOE allele status is a major influence on miRNA expression patterns in macrophages [125]. These authors reported that 152 miRNAs were differentially expressed in murine macrophages over-expressing ApoE4 compared with those over-expressing ApoE3. The differential elevation of mir-146a and miR-21 may be significant as they are associated with increased matrix metalloproteinase-9 (MMP-9) production and a corresponding increase in macrophage-associated tissue damage [125]. The upregulation of miR-146a may be of particular pathological relevance as this molecule may be upregulated by IL-1β, TNF-α or LPS, and increased activity of this miRNA is associated with increases in the activity of numerous inflammatory pathways in AD (reviewed in [129]).
Influence of TREM-2 Elevation in PMBCs
TREM-2, although better known as a regulator of microglial function as will be discussed below, also regulates TLR responses on dendritic cells. TREM2 upregulation in such cells appears to accelerate their maturation and trafficking to lymph nodes and sites of infection, as well as stimulating the differentiation of Th2 or Th17 cells, dependent on the nature and concentration of the antigen presented [130, 131]. The fact that TREM-2 acts as an ApoE receptor may also be of importance as this allows for an exaggerated effect of the ApoE4 protein in the context of dysfunctional TREM-2 receptors [132]. Moreover, a recent meta-analysis reported that increased methylation of the TREM2 promoter region appears to be an invariant feature in the brains of AD patients independently of age and sex [64]. Moreover, this increase in methylation is associated with a higher level of TREM-2 activity in the brains of AD patients compared with healthy controls [69]. Increased expression of TREM-2 receptors on peripheral leucocytes of AD and MCI patients, associated with reduced methylation in TREM2 intron 1, has been consistently reported [133,134,135]. Tan and colleagues have investigated the relationship between increased expression of TREM2 mRNA in the periphery in AD patients, and their study appears worthy of particular consideration as their results appear to emphasise the importance of peripheral abnormalities in the development of neuropathology in AD and partly to explain the relationship [135]. Briefly, these authors reported highly significant negative correlations (controlling for age, sex, ethnicity and APOE allele status) between, on the one hand, TREM2 mRNA expression (following amplification by real-time quantitative polymerase chain reaction (qPCR)), and, on the other hand, MMSE score residuals, episodic memory score residuals and Montreal Cognitive Assessment (MoCA) score residuals; there was also a negative correlation with right hippocampal volume and with the grey matter (GM) volumes of the frontal, temporal and parietal cortices [135]. Their analyses also revealed that, following a median split according to MoCA scores, compared with controls those AD patients in the lower group (MoCA scores ≤ 20) had higher TREM2 mRNA expression, which correlated with reduced volumes of total GM and right and left hippocampi [135].
Effect of PP2A Inhibition
Finally, it is also noteworthy that PP2A inhibition, which also appears to be a universal feature in AD patients [29], may lead to exacerbated PIC production by LPS-activated APCs [136, 137]. The mechanism underpinning this phenomenon has not been fully delineated but it appears to be associated with altered levels of histone post-translational methylation leading to increased transcription of TNFA (the TNF-α gene) and a general increase in inflammatory status [137]. These findings, allied to those discussed above, may well be important from the perspective of AD pathogenesis as the association between peripherally increased PICs and TREM-2 and increased AD risk and/or severity could be explained by the initiation and/or exacerbation of microglial activation, either as a result of high peripheral PIC levels and/or the egress of activated Th1 and/or Th17 cells into the CNS. Readers interested in the details of the mechanisms involved are referred to these reviews by Morris and colleagues [138] [139]. The pathological consequences of microglial activation and dysfunction and the putative role of these glial cells in the pathogenesis and pathophysiology of AD are discussed below.
Role of Microglia and Astrocytes
It should be stated at the outset that much of the data regarding the role of microglia in AD has been obtained from in vitro and non-human animal studies or AD patients post mortem, and their role is still a source of debate [140]. However, the use of in vivo neuroimaging techniques has consistently revealed a pattern of microglial activation consistent with an increased inflammatory status. For example, Parbo and colleagues reported the presence of increased cortical microglial activation in 85% of their MCI cohort [141]. Moreover, these authors noted that the patterns and extent of microglial activation correlated with the patterns and level of amyloid load in the parietal, frontal and temporal cortices [141]. Fan and co-workers also reported significantly elevated microglial activation at baseline in their AD participants, which increased in the majority of the patients over the course of the study [21]. Moreover, these authors reported that this longitudinal increase in microglial activation correlated with amyloid deposition and decline in regional cerebral metabolic rate over time [21]. In a later study, this team of authors investigated longitudinal changes in microglial activity in MCI and AD patients and reported a 36% increase in microglial activation over 14 months in the AD patients but an 18% decrease in the MCI patients for reasons which are not currently understood [20].
These findings are consistent with those of the work of other authors who have produced evidence suggesting that such microglial activation and subsequent production of cytokines, chemokines, nitric oxide (NO), prostaglandins, reactive oxygen species (ROS), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), and other mediators of inflammation and neurotoxicity also play a critical role in AD pathogenesis [142,143,144]. The weight of evidence suggests that microglia enter a hyper-reactive state in AD, and indeed other neurodegenerative conditions, and lose their normal beneficial function in maintaining neuronal homeostasis and phagocytic clearance during the progression of the illness [145], and ultimately adopt a neurotoxic or ‘primed’ phenotype [71, 146]. It has been proposed that this primed or hyper-responsive phenotype, which leads to an exaggerated production of neurotoxic substances following inflammatory activation, is the result of successive immune or inflammatory insults in the periphery [22, 147]. The activation of microglia, and the ultimate creation of a hyper-responsive phenotype, would also go some way to explaining the wealth of experimental data demonstrating that systemic inflammation, such as that resulting from pathogen invasion, can worsen the symptoms of AD or even trigger its development [142]. Unsurprisingly, there has been intensive research investigating the mechanisms underpinning microglial pathology in AD and currently, a great deal of research is focused on TREM-2, which is considered below.
Abnormalities in TREM-2 Levels and Function as a Source of Microglial Pathology
As mentioned above, a recent meta-analysis concluded that increased methylation of the TREM2 promoter region appears to be an invariant feature in the brains of AD patients independently of age and sex [64]; furthermore, this increase in methylation is associated with a higher level of TREM-2 activity in the brains of AD patients compared with healthy age- and sex-matched controls [69]. Moreover, there is a wealth of data demonstrating that functional variants of the TREM2 gene are strongly associated with an increased risk of late onset AD (LOAD) development [148, 149].
Increased TREM-2 activity in AD brains may be a significant source of pathology as this receptor plays a major role in regulating microglial activation and the inflammatory response following TLR activation, and facilitates immunologically silent phagocytosis of apoptotic neurones [150, 151]. Increased TREM2 expression in the temporal cortex of AD patients post mortem correlates significantly with increases in caspase-3 and phosphorylated-tau, and intense TREM-2 immunoreactivity is seen in microglia associated with amyloid plaques in regions of profound neuritic pathology [152]. This and other data have led to the proposal that TREM2 variants contribute to the development of Alzheimer’s disease via the downregulation of microglial Aβ phagocytic capability and dysregulation of microglial pro-inflammatory responses [151]. The relationship between TREM-2 and the development of a neuroinflammatory state appears to be complex and appears to involve improving microglial survival and metabolic performance as well as stimulating the release of PICs, ROS, reactive nitrogen species (RNS) and PGEs [60]. There is also some evidence to suggest that increasing levels of neuroinflammation provoke further upregulation of the TREM-2 receptor on activated microglia allowing for an upwards spiral of inflammation via a positive feedback loop [153]. In addition, TREM-2 acts as an ApoE receptor [132], as discussed above, and in this context, it is noteworthy that recent studies have established a relationship between TREM-2 and ApoE in the regulation of the microglial phenotype and the level of inflammatory mediators excreted by these glial cells following activation [154, 155]. In particular, evidence suggests that ApoE-mediated TREM-2 signalling provokes a change in microglial phenotype from tolerogenic to neurodegenerative following phagocytosis of apoptosed neurones in vivo [154] and the presence of the ApoE4 isoform is associated with higher levels of neuroinflammation in such circumstances by differentially increasing levels of TREM-2 [155].
TREM-2 activity is also intimately connected with microglial phagocytosis as discussed above and exerts its signalling effects via a multi-receptor complex with signalling adaptor molecule DNAX-activating protein of 12 kDa (DAP12) and dysfunction of this signalling axis may play a role in the impaired microglial phagocytosis repeatedly reported in AD brains. Briefly, under physiological conditions, heat shock protein-90 (HSP-90) engagement with TREM-2 regulates the immunologically silent microglial phagocytosis of apoptotic neurones via engagement with DAP12 [152]. The protective effect of TREM-2 against the development of LPS-mediated neuroinflammation would also appear to be mediated by this route [156]. Given such information, the existence of data suggesting that functional mutations in either protein can have adverse effects on microglial phenotype and function is unsurprising and may be one factor accounting for the impaired microglial phagocytosis which appears to be a feature of AD [157, 158].
The physical association between TREM-2 and DAP12 plays a vital role in determining the outcome of TREM-2 activation and in particular anti-inflammatory consequences are dependent on DAP12-mediated stabilisation of the C-terminal fragment of TREM-2 (CTF) and the loss of physical contact has pro-inflammatory consequences [156]. This is of importance as there is evidence to suggest that CTF accumulation in AD leads to disconnection between TREM-2 and DAP12, which could provide a mechanism to explain impaired phagocytosis and the pro-inflammatory consequences of TREM-2/DAP12 signalling in AD and a range of other neurodegenerative diseases [158,159,160].
Epigenetic Dysregulation of ANK1 as a Source of Microglial Pathology
The pivotal role of microglial pathology in the pathogenesis of AD has been further highlighted by research into the methylation status of the gene ANK1, the expression of which in AD brains in vivo appears to be confined to these glial cells [61]. Briefly, two independent research teams have reported the presence of a hypermethylated region in ANK1 and changes in ANK1 mRNA levels are associated with the geographical extent and overall burden of neuropathology in the entorhinal cortex, prefrontal cortex and superior temporal gyrus in symptomatic and pre-symptomatic AD patients in post mortem studies [65, 66]. These are important observations: they are relatively large studies and the methylation changes seen in asymptomatic patients are unlikely to be the product of disease pathology [161]. It should also be noted that the association between AD pathology and ANK1 methylation status may well be the most robust of all epigenetic and genetic associations with disease development reported thus far [61, 161].
The mechanisms underpinning this association are not understood, but they may be connected to altered expression of miR-486. ANK1 is a host gene for miR-486 [162] which in turn is a source of two mature miR-486 miRNAs, namely, miR-486-3p and miR-486-5p [163]. Importantly, ANK1 hypermethylation inhibits the transcription of miR-486 [164], which may have pathogenic consequences as suppression of this miRNA has pro-inflammatory consequences and is furthermore associated with increased cellular survival and proliferation [165, 166]. Furthermore, upregulation of miR-486 acts as a negative regulator of Akt (protein kinase B), mTOR and STAT3 (signal transducer and activator of transcription 3), all of which play major roles in microglial activation, proliferation and survival [167, 168].
mTOR plays a pivotal role in determining the inflammatory status and proliferation of microglia following PIC-mediated activation, which are both key determinants of neuroinflammation [169]. Akt upregulation is also a major driver of microglial activation and polarisation into the M1 phenotype [170]. STAT3 activation also plays a major role in determining the magnitude of the proliferative and inflammatory responses of activated microglia and, crucially, activation of this transcription factor also inhibits the microglial phagocytosis and clearance of Aβ in vivo [171, 172]; reviewed by [173]. Thus, it is conceivable that ANK1 hypermethylation accounts for the elevated mTOR and STAT3 signalling which has been repeatedly documented in the microglia of AD patients [172, 174,175,176].
Role of Aβ in Microglial Pathology
This is a well-documented area and has been considered by numerous authors (e.g. [15, 177]). It seems reasonable to propose that accumulating levels of Aβ as a result of impaired clearance would also play a role in maintaining microglia in a chronic state of activation following antigenic stimulation via engagement with TLR-2, which mediates antigenic stimulation of these glial cells by this peptide [178]. However, the capacity of Aβ to activate microglia in vivo has not been demonstrated and several authors have noted that human brains with very high Aβ loads reveal an absence of microglial activation [140, 179].
Interactions Between Microglia and Astrocytes and Exacerbated Neuroinflammation
Early AD is characterised by astroglial atrophy leading to impaired blood-brain barrier (BBB) structure and function, synaptic dysfunction, mitochondrial dysfunction and impaired neuronal homeostasis [180,181,182]. Later disease is associated with reactive astrogliosis where activated astrocytes make an independent contribution to increasing neuroinflammation and neurotoxicity [180, 181]. Astrocytes make many contributions towards brain homeostasis in the context of AD, including regulation of oxygen and energy delivery to neurones, regulation of cholesterol delivery to neurones, neurotransmission, and immune and inflammatory responses in the CNS, in a similar manner to its activity in the periphery discussed above [182]. This is highly relevant because astrocytes are by far and away the largest producers of ApoE in the brain and ApoE4 is known to impair BBB function to a greater extent than other ApoE isoforms [183, 184]. Activated astrocytes are also a source of Aβ42 protofibrils, likely synthesised by the actions of PICs [185, 186]. There is also some evidence that reactive astrocytes in AD not only secrete increased levels of Aβ42 as discussed above but also conspire with adjacent neurones to promote further increases in Aβ42 and levels of ptau over a wider geographical area as the disease progresses [187].
There are a number of mechanisms which could account for the greater levels of peripheral inflammation and neuroinflammation that occurs in AD patients than in age- and sex-matched controls, which in turn appear to make a significant contribution to the development of AD, and it is certainly plausible that the development of AD begins with pathology in the periphery. It should also be noted that, while the data reviewed above focus heavily on inflammation, this phenomenon is invariably accompanied by oxidative stress [188, 189]. Hence, the mechanisms potentially explaining differentially elevated inflammation in the brain and periphery of AD patients also potentially explain elevated oxidative stress in both compartments. This is an important point as the remainder of the paper focuses on the third research question, namely, whether differentially elevated inflammation and oxidative stress in the brain and periphery of AD patients is sufficient to explain impaired mitochondrial function, synaptic dysfunction, PPA2 inhibition, elevated mTOR, elevated GSK-3, impaired macro- and microautophagy, decreased proteasome function, increased iron accumulation and transition metal dyshomeostasis reported in AD patients compared with age- and sex-matched controls.
Evidence and Consequences of Chronic Oxidative Stress in AD
Evidence of Increased Oxidative Stress in the Brain and Periphery in AD Patients
Surrogate markers of protein oxidation, lipid peroxidation and oxidative damage to DNA, such as protein carbonyls, 3-nitrotyrosine, malondialdehyde (MDA), 4-hydroxynonenal, F2-isoprostanes, 8-hydroxydeoxyguanosine (8-OHdG) and 8-hydroxyguanosine, are elevated in the cerebrospinal fluid, the brain and peripheral cells of patients with AD [16, 190, 191]. Damaged proteins, lipids, RNA and DNA in regions of the brain associated with cognitive function are also a reproducible finding in AD and MCI and are held to have a functional role in disease pathogenesis [192, 193].
Effect of Oxidative Stress on the Development of Amyloid and Tau Pathology
Oxidative stress not only impairs the activity of α-secretase but also enhances the activation and expression of β- and γ-secretase [194, 195]. The oxidative stress-driven stimulation of β-secretase 1 (BACE1) and presenilin-1 (PS1) activities, and the activation of γ-secretase, are dependent on the NF-κB and activator protein 1 (AP-1)-induced activation of the c-Jun N-terminal kinase (JNK) pathway [196, 197]. In essence, the promoter region of the BACE1 gene hosts binding sites for the redox-sensitive AP-1 and NF-κB; the activation of which in an environment of chronic oxidative stress explains the enhanced transcription of BACE1 [198], elevated JNK signalling [16], increased expression of BACE1 and increased PS1 activity, which have been detected in AD brains [199,200,201]. Hence, NF-κB- and AP-1-induced activation of JNK signalling, and consequent upregulation of BACE1 and PSEN1 (which encodes PS1), likely could lead to increased Aβ production and possibly an exacerbation of cognitive decline and neuronal apoptosis in AD [16, 200].
There is also an accumulating body of evidence indicating that chronic oxidative stress has a direct causal role in tau phosphorylation [202,203,204]. The mechanisms underpinning these observations remain to be fully elucidated but the weight of evidence implicates elevated levels of fatty acids and p38 signalling [204,205,206].
Other Pathological Consequences of Elevated Oxidative Stress
Signs of oxidative and nitrosative damage to proteins and lipids are amongst the earliest indicators of early disease and occur before evidence of Aβ accumulation [28]. A study comparing F2-isoprostane levels in the frontal poles of AD brains with the same regions from brains of patients with schizophrenia and Parkinson’s disease (PD) reported no differences between PD, schizophrenia and controls, but the levels were significantly increased in AD which potentially allows for higher levels of oxidative stress in those brain areas as a unique contribution to the pathogenesis of the illness [207].
Oxidative stress has also been associated with APOE status in AD patients and interestingly also in healthy subjects [28]. In particular, the APOE ε4-positive status is associated with a relatively higher level of oxidative stress and diminished antioxidant enzyme activity in the hippocampus of AD patients [208]. The association with APOE status is not surprising as ApoE is a key player in organising cellular antioxidant responses [209]. The levels of oxidative stress in peripheral lymphocytes are also higher in AD patients with at least one copy of the APOE ε4 allele [210]. It is also of interest that APOE ε4 directly facilitates the phosphorylation of tau, potentially increasing the filamentous load of this protein in the brain in AD [211].
Oxidative Stress and the Development of Mitochondrial Dysfunction in AD Patients
Extensive studies have demonstrated that mitochondrial dysfunction is an important factor involved in the pathogenesis of AD and is apparent in the earliest stages of the disease both in the brain and the periphery [19, 212]. Several studies have identified structural and functional mitochondrial abnormalities in hippocampal neurones of AD patients compared with age- and sex-matched controls [213,214,215,216]. Such abnormalities include a significant reduction in mitochondrial numbers and exaggerated levels of oxidised mitochondrial DNA (mtDNA) and nitrated proteins in the cytoplasm in a pattern suggestive of impaired mitophagy or fission dynamics [215,216,217]. These mitochondrial abnormalities are accompanied by oxidative damage marked by 8-hydroxyguanosine and nitrotyrosine, indicating that the mitochondria are damaged by ROS and RNS during disease progression [213, 218, 219].
Several authors have reported decreased mitochondrial complex IV activity in the frontal cortex of AD patients and this phenomenon leads to increased ROS production and depleted adenosine triphosphate (ATP) production, contributing to neuronal dysfunction and, ultimately, degeneration [213, 214, 217]. Systemic mitochondrial dysfunction is also apparent in all phases of the illness, as evidenced by impaired mitochondrial electron transport chain (ETC) activity and depleted basal mitochondrial membrane potential, seen in PMBCs of patients with AD and MCI [220,221,222]. In this context, it is noteworthy that high levels of NO have a well-documented inhibitory effect on ETC enzymes as a result of S-nitrosylation of key functional cysteine residues in their catalytic sites (reviewed in [223]). Oxidative and nitrosative stress can also lead to oxidative damage to key enzymes of the tricarboxylic acid (Kreb’s) cycle, leading to their inactivation, which exerts a range of unfavourable effects on cellular bioenergetics. These are well documented phenomena and will not be considered further here. The interested reader is referred to the works of Morris, Maes and Praticò [207, 224] for details of these mechanisms. It is, however, worthy of note that mitochondrial dysfunction leads to dramatically elevated levels of ROS and NO production, which further compromise mitochondrial function, leading to a vicious spiral of mitochondrial damage and bioenergetics failure [225, 226].
There is accumulating evidence that APOE status has an effect on mitochondrial function in at least some patients with AD. Gibson and colleagues reported that mitochondrial dysfunction was more common in the brains of AD patients with the APOE ε4 allele [227]. The mechanisms explaining this association are not completely understood but there is some evidence to suggest that the neurotoxicity stems at least in part from the entry of ApoE4 isoform fragments into the cytosol and ultimately into mitochondrial membranes [184]. Once in situ, this lipoprotein induces mitochondrial dysfunction by binding to the α- and β-subunits of the mitochondrial F1-ATPase and disrupting mitochondrial membrane integrity leading to dissipation of the trans-membrane potential difference [184, 191]. There is also some evidence that the APOE ε4 allele and mtDNA haplogroups are cooperative variables in the sporadic form of AD [228].
In addition, accumulating data indicate that changes in methylation status of the promoter region of the APOE gene in AD patients can have a direct influence on mitochondrial function and indeed the development of mitochondrial pathology [67, 68]. In brief, the methylation status of a CGI in the 3′ region, 2.6 kb downstream of the APOE promoter, modulates APOE expression. Moreover, common APOE SNPs reside in this region and can regulate levels of methylation and transcription in an allele-specific manner with ε4 having a greater effect than ε3. These methylation changes not only influence the transcription of APOE but also affect that of TOMM40 encoding the mitochondrial protein translocase of outer mitochondrial membrane 40 homolog (TOMM40), which plays an essential role in the importation of proteins into the organelle [67, 68]. This is significant given that a recent study has reported reduced levels of TOMM40 in the brains of AD patients which correlated with the extent of cognitive decline [229] and the results of a large meta-analysis involving 10,358 AD cases and 18,157 healthy controls which concluded that the TOMM40 rs2075650 polymorphism was associated with an increased risk of disease development (odds ratio 4.178) [230]. The mechanisms underpinning reduced TOMM40 expression and/or conformational changes to this protein and the development of AD and other neurodegenerative diseases are discussed by Gottschalk and colleagues [231]. Lastly, there is evidence that mitochondrial dysfunction might be worsened by neuronal accumulation of oligomeric Aβ (OAβ).
Oxidative Stress and the Development of Synaptic Dysfunction
Numerous research teams have adduced evidence supporting a direct causal relationship between oxidative stress and the development of synaptic dysfunction in AD [232]; reviewed by [233]. This is also true of mitochondrial dysfunction and glucose hypometabolism which is apparent in the posterior cingulate cortex and other AD-vulnerable brain regions in MCI patients and healthy adult carriers of APOE ε4 many years or even decades before the development of clinical symptoms and, crucially, before any discernible evidence of tau or Aβ pathology [234, 235]; reviewed by [236]. The origin of glucose hypometabolism, which appears to be an invariant feature in the brains of AD patients [40], is a subject of debate with some authors suggesting that this phenomenon is secondary to mitochondrial dysfunction [44] while others cite as the cause of brain insulin resistance, which is also an invariant feature in AD patients [41]. It is also of interest that the insulin resistance seen in AD patients could be the result of chronic oxidative stress [45, 237], indirectly associating chronic oxidative stress with the development of glucose hypometabolism [238, 239].
The association between impaired mitochondrial performance and the development of synaptic dysfunction is not unexpected as these organelles are involved in every stage of neurotransmission including the synthesis and storage of neurotransmitters, the trafficking and recycling of synaptic vesicles (SVs), presynaptic neurotransmitter release, neurotransmitter synthesis, calcium ion homeostasis as well as supplying ATP and regulating levels of ROS [240,241,242].
Mechanisms underpinning the detrimental effects of excessive ROS levels on synaptic function are underpinned by oxidation of cytosolic and membrane proteins and peroxidation of membrane lipids [243, 244]. For example, several research teams have reported that lipid peroxidation in presynaptic membranes impedes fusion pore opening, thereby restricting SV exocytosis, resulting in the abnormal retention of SVs within presynaptic active zones [245, 246]. The last phenomenon may go some way to explaining the presence of data demonstrating attenuation of synaptic neurotransmission and long-term potentiation (LTP) by high levels of ROS (reviewed by [247]).
More specifically, increasing levels of ROS and RNS could account for the progressive loss of cholinergic neurones and increasing dysfunction of cholinergic neurotransmission which are characteristic of AD patients as their disease progresses [248]. For example, the enzyme choline acetyltransferase (ChAT) and the high-affinity choline transporter (CHT), the enzymes responsible for synthesising and recycling acetylcholine (ACh), respectively, are vulnerable to post-translational modifications leading to compromised trafficking and protein-protein interactions or indeed inactivation by the effects of ROS and ROS owing to a high density of essential cysteine residues in enzyme catalytic sites (reviewed by [249]). Moreover, acetylcholinesterase (AChE), an enzyme responsible for ACh hydrolysis in the synaptic cleft, is also prone to inhibition in such an environment [250, 251]. Furthermore, there is a considerable body of evidence indicating that cholinergic neurones are highly susceptible to apoptotic or necrotic death in an environment of excessive nitrosative and oxidative stress [252, 253] via mechanisms detailed in a recent paper by Morris and fellow workers [139]. Readers interested in a detailed consideration of cholinergic neurotransmission and the role of the molecular players described above in the context of AD are invited to consult an excellent review by Ferreira-Vieira and fellow workers [248].
The existence of synaptic dysfunction in AD may also be influenced by inhibition of glutamatergic N-methyl-d-aspartate (NMDA) receptors, which has been repeatedly reported in AD patients [254], via oxidation of cysteine groups on key structural and functional proteins leading to profound changes in conformation and function [223, 255]. NMDA receptor function can also be compromised by high levels of NO through hypernitrosylation of key receptor subunits [226] and via the formation of the excessively damaging peroxynitrite [256].
While the association between increased oxidative stress and increasing bioenergetic dysfunction, as evidenced by increasing glucose hypometabolism, increased lactate and pyruvate and the development of increasing synaptic dysfunction seen in preclinical AD and APOE ε4 carriers, is not associated with Aβ accumulation [257] (reviewed by [258]), recent research suggests that this might not be the case for tau deposition although findings are mixed [259,260,261]. For example, Bischof and others and Kang et al. reported a positive correlation between tau deposition and glucose hypometabolism in cross-sectional studies [259, 261]. However, Chiotis and colleagues concluded that increases in glucose hypometabolism were not associated with increased tau deposition in a large longitudinal study [260].
Increased Oxidative Stress and Altered GSK-3 Activity in AD Patients
Prolonged and severe oxidative stress leads to the activation of GSK-3 [262,263,264]; its physiological levels of expression and activity play an indispensable role in the regulation of synaptic function and other aspects of neurotransmission as well as levels of tau phosphorylation [12, 265]. Given this information, the presence of data implicating dysregulation in the activity of the two isoforms of this kinase as one cause of synaptic dysfunction in MCI and AD is unsurprising (reviewed by [266]).
The weight of direct and indirect evidence suggests that GSK-3 production is increased in the hippocampus and frontal cortex of AD patients [267, 268] and in post-synaptosomal supernatants derived from AD brain [269]. Active GSK-3 also appears in neurones before the development of NFTs and it co-localises with dystrophic neurites and NFTs in later stages of the disease [269,270,271]. GSK-3 is also upregulated in peripheral lymphocytes in MCI and AD [272]. The importance of GSK-3 in the pathogenesis of AD has been emphasised by reports that a GSK3B polymorphism is a significant risk factor for the development of LOAD [273]. Both isoforms of GSK-3 (GSK-3β and GSK-3α) appear to induce the hyperphosphorylation of tau [274, 275], but GSK-3α alone regulates the cleavage of APP and would appear to exert this role in the very early phase of the disease [276,277,278]. However, increased GSK-3β signalling also seems to play a pathological role in amyloid processing as such signalling increases BACE1 expression, thereby facilitating the increased production of Aβ [279]. Conversely, and unsurprisingly, inhibition of this enzyme leads to a reduction in Aβ production [279]. There is evidence that GSK-3α enhances the activity of the γ-secretase complex [277] and may act to downregulate the activity of α-secretase [280]. There is also accumulating evidence, albeit in vitro, demonstrating that ApoE4 increases GSK3B expression, potentially leading to the exacerbation of pathology associated with the activation and upregulation of this kinase [281, 282].
Oxidative Stress mTOR Activation and Impaired Autophagy and UPS Clearance
Background
Autophagy encompasses a series of pathways by which damaged cytosolic components are transferred to lysosomes and subjected to enzymatic degradation in an immunologically silent manner (reviewed in [283]). There are three recognised subgroups of autophagy, namely macroautophagy, the dominant form in human cells, microautophagy and chaperone-mediated autophagy. Readers interested in a detailed examination of these processes and the differences and similarities between them are referred to a comprehensive review by Yu and others [284]. The UPS, on the other hand, is based on the receipt of ubiquitin-tagged oxidatively damaged and/or misfolded proteins by the barrel-shaped 26S proteasome, composed of multiple protein subunits, via a narrow opening (see [285]). Once ensconced, such proteins are subjected to a range of proteolytic enzymes, ultimately producing ubiquitin-tagged monomers [286].
The autophagic process is upregulated in the brains of AD patients, most notably in the hippocampus and other areas of the brain associated with AD pathology [287]. These observations may be significant in terms of differentiating AD from normal ageing, as there is copious evidence that the autophagic process is downregulated in normal ageing [288, 289]. However, despite the transcriptional upregulation of autophagy seen in AD patients, the weight of evidence indicates that autophagic lysosomal clearance is dysregulated and defective in the hippocampus of AD patients, even in those in the very early stages of their disease [287] [24].
The activity of the UPS is also impaired in the hippocampus and other disease-susceptible brain regions in AD patients, but apparently not in brain regions not associated with AD-specific neurodegenerative pathology (reviewed by [285, 286]). It would also appear that changes in the protein composition of the 26S proteasome and impaired activity of ubiquitin C-terminal hydrolase L1 (UCH-L1), a deubiquitinating enzyme responsible for the production of ubiquitin-tagged monomers, may be a characteristic of AD [290, 291]. From the perspective of this paper, it is especially noteworthy that downregulation of this enzyme appears to be the result of oxidative damage and occurs in AD patients many years before any evidence of amyloid plaques or NFTs [286, 291].
Oxidative Stress and mTOR Activity in AD
mTOR is recognised as one of the master regulators of cellular metabolism in general and in autophagic processes in particular [292]. Readers interested in the many homeostatic roles, biochemistry and mechanistic actions of the two mTORC (mTOR complex) isoforms are referred to excellent reviews by Laplante and Sabatini [293, 294].
The weight of evidence suggests that mTOR activity is increased in the temporal cortex and hippocampus of AD patients [295, 296], and an activated but dysregulated Akt/mTOR signalling pathway in the hippocampus would appear to be a universal feature of AD and MCI (reviewed by [297]). It is noteworthy that MTOR expression is normally increasingly inhibited in the ageing brain [298, 299], and hence the existence of elevated mTOR activity in the hippocampus of AD patients could be a factor underpinning dysfunctional autophagic lysosomal clearance in that region of the brain, as discussed above. From the wider perspective of AD pathology, mTOR has several roles, such as the regulation of many aspects of synaptic function and protein aggregation, and is known to promote ptau and tau dyshomeostasis [300,301,302,303]. Some authors also propose that intricate molecular interactions between Aβ, tau and mTOR exacerbate the rate of cognitive decline [304, 305]. Elevated mTOR signalling is also relevant from the perspective of the more ‘generic’ elements involved in disease pathogenesis as this kinase regulates mitochondrial function [306], immune cell homeostasis [307] and levels of oxidative stress [308]. It is also of interest that mTOR activity in AD does not appear to be modified by APOE allele status, which hints that this molecule could play a unique role in AD pathology which is not seen in normal ageing [300].
Oxidative Stress and Compromised UPS Function and Structure
Initially, increased levels of oxidative stress provoke a defensive response whereby the 26S proteasome dissociates into its 20S and 19S subunits, with the former being resistant to oxidative damage and thus responsible for protein degradation in this changed environment [309,310,311]. This adaptive response has limitations however, and during the development of chronic oxidative stress, the 20S subunit as well as the 26S proteasome may also become deactivated [309, 312], leading to the accumulation of insoluble covalently crosslinked proteins which can further inhibit the proteasome [310, 313]. Proteasomal dysfunction can lead to decreased degradation of misfolded proteins, thus resulting in accumulation of oxidised proteins and subsequent protein aggregation. Protein aggregates can then feedback, further to inhibit proteasomal activities, generate additional cellular stress and lead to cytotoxicity [309, 310, 314]. Additionally, oxidatively modified proteins may impair the cellular machinery of autophagic degradation [314, 315]. Reactive species can damage the lysosomal membrane and crosslinked membrane proteins, resulting in cytosolic leakage of lysosomal hydrolases [315, 316].
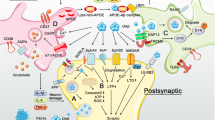
When considered as a whole, the data demonstrate that chronic oxidative stress impairs autophagy by provoking unfavourable changes in autophagic degradation, inhibition of lysosomal enzyme function and lysosomal membrane damage [317]. Furthermore, some oxidatively modified aggregated species are resistant to degradation by proteases and accumulate within lysosomes. There, the non-degraded proteins become a potential new source of reactive species, further damaging the lysosomal membrane [318]. This oxidative damage to lysosomal lipid membranes can be exacerbated by high levels of iron seen in AD patients, which increase the sensitivity of these membranes to oxidative damage to the point of inducing apoptotic or necrotic cell death resulting from lysosomal rupture and release of toxic hydroxylases, calpains and redox-active iron into the cytoplasm [139]. There is a growing appreciation that the role of redox-active iron and iron dyshomeostasis as a driver of neuropathology in AD may be pivotal in AD both as a source of increasing oxidative stress, via hydroxyl production through the Fenton reaction, and in the development of amyloid- and tau-related pathology. Hence, the role of oxidative stress in the development of iron dyshomeostasis and accumulation in the brains of AD patients and the pathological consequences of this phenomenon is the focus of the remainder of this paper. Understanding the content below requires some knowledge of the factors involved in maintaining iron homeostasis, which are depicted in Fig. 3 and summarised in the accompanying legend.
Iron homeostasis in neurones. Neurones and glial cells can uptake iron bound to transferrin (TBI), or bound to other molecules such as citrate and ATP secreted by astrocytes (NTBI). Neuronal uptake of TBI is enabled by the transferrin receptor located at the cell membrane and the uptake of NTBI in inflammatory conditions is probably enabled by DMT1. DMT1 and TfR1 complexes are internalised via endocytosis, ultimately resulting in the release of redox-active iron (Fe(II)) into the cytosol and the return of other molecules in the complexes to the plasma membrane. Once in the cytosol, Fe(II) can be utilised for various essential metabolic processes such as the synthesis of iron-sulphur proteins, or sequestrated by cytosolic ferritin and mitochondrial ferritin (FtMt), which offers protection against the advent of the Fenton reaction. Iron is removed from neurones by ferroportin, supported by the multi-copper-containing ferroxidase caeruloplasmin and sAPP, which both act to stabilise ferroportin at the cell surface
Oxidative Stress and Iron Accumulation in AD
Evidence of Iron Dyshomeostasis in AD Patients
Sophisticated MRI approaches have allowed the detection of increased iron levels in the brains of AD patients, most notably in the putamen and in posterior GM and white matter regions [319,320,321]. Elevated iron levels in the cortex and cerebellum are also a commonly reported phenomenon in MCI patients [322]. Levels of intracellular iron are subject to strict homeostatic regulation at the translational and transcriptional levels.
Transcriptional Regulation of Iron Homeostasis
Regulation at the transcriptional level is mediated by interplay between the iron transport exporter protein ferroportin-1 (fpn-1) and the peptide hormone hepcidin, whereby increased activity of the latter leads to a reduction in the activity and levels of the former, hence reducing the cellular export of iron [323, 324]. Crucially, hepcidin synthesis is upregulated in an environment of chronic oxidative stress and neuroinflammation as a result of elevated H2O2 [325] and/or IL-6-activated STAT3 [326,327,328,329]. In fact, lower H2O2 concentrations (in the range of the levels observed during inflammation) require STAT3 phosphorylation to induce hepcidin and may, synergistically with IL-6, stimulate hepcidin [325]. This is clearly one mechanism underpinning the adverse effect of neuroinflammation and oxidative stress on iron accumulation in the CNS. Several authors have also reported that upregulation of divalent metal transporter 1 (DMT1) on the surface of neurones and glial cells results from the release of TNF-α, IL-1β, IL-6 and NO by LPS-activated microglia [330,331,332]. Importantly, the release of PICs from activated microglia, most notably IL-6, also leads to increases of hepcidin and reduction of ferroportin in neurones, which supplies a mechanism allowing increasing levels of neuronal iron accumulation over time in an environment of neuroinflammation [330, 331, 333].
Regulation of Iron Homeostasis at the Translational Level
Regulation of iron homeostasis and the translational level are governed by iron regulatory proteins (IRP) 1 and 2, which can bind to iron response elements (IRE) in the 5′ or 3′ untranslated regions (UTRs) of the mRNA sequences responsible for the production of proteins involved in iron homeostasis. This interplay is described as the IRP/IRE system (reviewed by [334]). The organisation and function of this system is depicted in Fig. 4 and explained in the accompanying legend.
Post-transcriptional control of iron homeostasis in neurones and glial cells. Binding of IRP1 and IRP2 to IRE in the 5′-UTR of mRNAs encoding ferritin and ferroportin represses translation, while binding of IRP1 and IRP2 to IRE in the 3′-UTR of mRNAs encoding TfR1 and DMT1 stabilises the mRNA resulting in efficient translation. In an environment of increasing oxidative stress, IRP2 is degraded while IRP1-mRNA binding is enhanced, which inhibits the synthesis of ferritin, ferroportin and APP while simultaneously upregulating the production DMT1 and TfR1. The cumulative effect of these activities is significantly increased iron
Detrimental Effect of Oxidative Stress on the IRP/IRE System
In an environment of increasing oxidative stress, IRP1 RNA binding is enhanced which inhibits the synthesis of ferritin, ferroportin and APP while concomitantly upregulating the production of DMT1 and transferrin receptor (TfR1) [335]. The cumulative effects of these activities are significantly increased iron uptake, a major reduction in iron sequestration and increased uptake of transferrin-bound iron (TBI) and non-transferrin-bound iron (NTBI) [335,336,337,338,339]. NO, and indeed peroxynitrite, also increase the mRNA binding of IRP1-IRE sequences [340, 341]. Elevated levels of NO also promote the degradation of IRP2 via a number of mechanisms including S-nitrosylation of crucial cysteine residues [342, 343], leaving IRP1 as the sole IRP regulating iron levels in brain cells in an environment of chronic oxidative and nitrosative stress. It is also interesting to note that IRP1-IRE complexes appear to be the only active complexes in the brains of AD patients [344]. In addition, it is noteworthy that recent findings indicate that increased APP activity and aggressive Aβ deposition seen in AD patients result, at least in part, from iron accumulation and dysfunctional IRP-IRE signalling [345, 346]. The role of iron accumulation in increased APP production is further highlighted by evidence demonstrating that iron chelation selectively downregulates APP mRNA production [347, 348].
Effect of Elevated Fe(III) and Fe(II) on the Development of Amyloid and Tau Pathology
Effect on APP Processing
APP translation is regulated by IL-1 activity and the IRE element in the 5′ UTR of APP mRNA. This IRE region interacts with IRP1 in human brain cortical tissue [348, 349]. Therefore, increasing levels of iron can stimulate the translation of APP by provoking the dissociation of IRP1 as described above [348]. Hence, prolonged increases in neural iron have the effect of increasing the amount of APP available for amyloidogenic processing and Aβ production. In addition, elevated iron also reduces the α-secretase cleavage of APP and favours proteolysis by β-secretase [350, 351]. Mechanistically, this phenomenon stems from the capacity of iron to reduce the transcription of the proconvertase furin. Under physiological conditions, furin initiates cleavage of A dysintegrin and metalloproteases 10 (ADAM10) and TNF-α-converting enzyme (TACE) and increases the activity of α-secretase and hence the production of α-secretase-derived secreted form of APP (sAPPα) [352]. However, iron-induced suppression of furin transcription enhances the activity of β-secretase activity, thereby stimulating the amyloidogenic pathway and thus the production of Aβ1–42 [351, 353]. The plausibility of this mechanism in vivo is further reinforced by evidence demonstrating that furin levels are reduced in the brains of AD patients [352].
Effect of Elevated Fe(III) and Fe(II) on Amyloid Plaque Formation
There is some evidence to suggest that the initial seeding of Aβ42 plaques with Fe(III) may be beneficial by facilitating the export of excessive insoluble iron via microglial phagocytosis and subsequent lysosomal degradation [354]. However, several research teams have produced preclinical data demonstrating that prolonged association of Fe(III) with Aβ1–42 leads to the reduction of the former and increased levels of redox-active Fe(II) [355, 356]. These findings have been recently reproduced in vivo in cortical tissue of AD transgenic mice [357]. Furthermore, these authors reported a direct correlation between elevated Fe(II) levels resulting from the reduction of Fe(III) by Aβ1–42 and pathological changes in plaque morphology particularly with regard to the protein/fibril density of fibrillar fragments and diffuse plaques [357]. The formation of Fe(II)/Aβ complexes in AD patients is important from a pathological perspective as Fe(II) has the capacity to interact with Aβ amino acids, subsequently conferring longitudinal changes to the normal patterns of amyloid formations [358, 359]. In brief, the interactions between Fe(III) and Fe(II), on the one hand, and APP and Aβ, on the other hand, influence the speed and extent of Aβ aggregation into fibrillary structures [360, 361]. More specifically, the weight of evidence suggests that when enough amyloid deposition has occurred, toxic oligomeric formations can propagate in a nonlinear amyloidogenic positive feedback loop, bypassing the normal requirement for amyloid monomers to form dimers [362], thereby accelerating Aβ aggregation, oligomerisation and amyloidogenesis [363, 364]. The role of iron in this process may be of paramount importance as there is evidence to suggest that Aβ plaques may not be neurotoxic in the absence of iron and that the oxidative and peroxidative damage to proteins and lipids associated with Aβ stems from its high affinity with iron and its capacity to reduce Fe(III) to Fe(II) thereby providing a redox-active Aβ-iron complex capable of producing destructive hydroxyl radicals in association with elevated hydrogen peroxide produced by soluble Aβ1–42 [190] reviewed by [365]. The indispensable role of elevated Fe(II) and Fe(III) as drivers of amyloid pathology is further supported by evidence obtained from rodent models of AD demonstrating that iron chelation can prevent Aβ aggregation and reverse memory loss [363, 366].
Effect of Elevated Fe(III) and Fe(II) on Tau Hyperphosphorylation and NFT Formation
Fe(II) can induce tau hyperphosphorylation [367, 368] via a mechanism involving the activation of the MAPK pathway and the extracellular signal-regulated kinase 1/2 (Erk1/2) pathway [369, 370]. This effect may be inhibited in vivo via the use of the iron chelator deferoxamine [368, 371]. This may induce a cascade of self-amplifying pathology as the hyperphosphorylation of tau and the formation of NFTs in the brains of AD patients results in an increased production of haem oxygenase-1 (HO-1) [372, 373] which provokes the release of Fe(II) [374], which may exacerbate ROS production via Fenton chemistry [353, 375]. Increasing levels of ROS may also explain the elevations in cytosolic copper and zinc seen in AD patients, which would occur as a result of oxidation of binding-thiol groups in their sequestration partner metallothionein [376, 377]; reviewed by [378]. It is also noteworthy that high levels of oxidative stress in neurones would be expected to inhibit PP2A [30, 379] and such inhibition could well add to the neurotoxic milieu, as reduced PPA2 activity is associated with neuronal death via a mechanism involving the activation of MAPK [380]. The pathological role of PP2A inactivation in the neurones of advanced AD patients may well be underestimated as there is evidence suggesting that impaired signalling of this phosphatase is a major element underpinning the hyperphosphorylation of tau in Parkinsonian dementia, often described as ‘a classical tauopathy’ [381].
The Function of the BBB in This Model
Several authors have reported reduced expression of adhesion molecules and tight junction proteins in BBB endothelial cells combined with a dysfunctional and/or disrupted neurovascular unit in AD patients with early disease long before the occurrence of dementia and in the absence of neurodegeneration and brain atrophy [382,383,384]. Importantly, such damage may result from the presence of prolonged systemic inflammation and elevated levels of PICs, which increase the permeability of tight junctions by decreasing levels of glycocalyx and other adhesion molecules, as well as causing endothelial cell damage and disruption of the of glia limitans [385, 386]. It is important to note that such damage may result from PICs in the systemic circulation or following activation of microglia and astrocytes in the brain and the latter phenomenon goes some way to explaining the dysfunction of the neurovascular unit seen in early AD patients described above [382, 385, 386].
Other Neurodegenerative Disorders
This peripheral model may help explain other neurodegenerative disorders besides AD. Numerous in vivo studies have demonstrated a dysfunctional or disrupted BBB and neurovascular unit in other neurological diseases such as PD [387, 388]. This is of interest as a recent meta-analysis has confirmed the presence of peripheral inflammation and elevated PICs in PD patients [389]. Moreover, the development of BBB disruption and the subsequent egress of activated T cells and other lymphocytes into the CNS further exacerbating microglial activation have been established as a causative factor in the pathogenesis of the illness [390]. The ultimate cause of chronic peripheral inflammation in PD patients is not entirely understood and may be multifactorial. However, it seems reasonable to conclude that pesticide exposure and perhaps a history of head trauma may be involved, as both factors appear to play as a causative role in the development of the illness [391, 392].
Conclusion
In conclusion, it has been shown that the presence of the APOE ε4 allele, and epigenetic dysregulation, including increased DNA methylation and altered miRNA expression, could explain increased levels of peripheral and central inflammation and oxidative stress in AD. Furthermore, this increased oxidative stress and inflammation could originate in the periphery rather than in the CNS itself. Finally, molecular neurobiological mechanisms have been adduced which explain how the initial development of elevated peripheral and central inflammation and oxidative stress in the context of genetic and epigenetic abnormalities could explain the development of AD.
References
Reddy PH, Beal MF (2008) Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med 14(2):45–53. https://doi.org/10.1016/j.molmed.2007.12.002
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-β in Alzheimer’s disease. Nat Rev Neurosci 8(7):499–509. https://doi.org/10.1038/nrn2168
Mattson MP (2004) Pathways towards and away from Alzheimer’s disease. Nature 430(7000):631–639. https://doi.org/10.1038/nature02621
O’Brien RJ, Wong PC (2011) Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci 34(1):185–204. https://doi.org/10.1146/annurev-neuro-061010-113613
Zhang YW, Thompson R, Zhang H, Xu H (2011) APP processing in Alzheimer’s disease. Mol Brain 4:3. https://doi.org/10.1186/1756-6606-4-3
Zhang X, Song W (2013) The role of APP and BACE1 trafficking in APP processing and amyloid-β generation. Alzheimers Res Ther 5(5):46. https://doi.org/10.1186/alzrt211
Konietzko U (2012) AICD nuclear signaling and its possible contribution to Alzheimers disease. Curr Alzheimer Res 9(2):200–216. https://doi.org/10.2174/156720512799361673
De-Paula VJ, Radanovic M, Diniz BS, Forlenza OV (2012) Alzheimer’s disease. Protein aggregation and fibrillogenesis in cerebral and systemic amyloid disease Springer Netherlands doi:https://doi.org/10.1007/978-94-007-5416-4_14
Morris GP, Clark IA, Vissel B (2014) Inconsistencies and controversies surrounding the amyloid hypothesis of Alzheimer’s disease. Acta Neuropathol Commun 2:135. https://doi.org/10.1186/s40478-014-0135-5
Chetelat G, La Joie R, Villain N, Perrotin A, de La Sayette V, Eustache F, Vandenberghe R (2013) Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. Neuroimage Clin 2:356–365. https://doi.org/10.1016/j.nicl.2013.02.006
Greenough MA, Camakaris J, Bush AI (2013) Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem Int 62(5):540–555. https://doi.org/10.1016/j.neuint.2012.08.014
Llorens-MarÃtin Ma, Jurado Jn, Hernández Fl, Ãvila Js (2014) GSK-3Î2, a pivotal kinase in Alzheimer disease. Front Mol Neurosci 7. doi:https://doi.org/10.3389/fnmol.2014.00046
Skaper SD (2012) Alzheimer’s disease and amyloid: culprit or coincidence? Int Rev Neurobiol Elsevier. https://doi.org/10.1016/b978-0-12-386986-9.00011-9
Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW (2011) Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol 10(9):785–796. https://doi.org/10.1016/s1474-4422(11)70156-9
Morris G, Berk M (2016) The putative use of lithium in Alzheimer’s disease. Curr Alzheimer Res 13(8):853–861
Zhao Y, Zhao B (2013) Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Med Cell Longev 2013:10. https://doi.org/10.1155/2013/316523
Padurariu M, Ciobica A, Lefter R, Serban IL, Stefanescu C, Chirita R (2013) The oxidative stress hypothesis in Alzheimer’s disease. Psychiatr Danub 25(4):401–409
Swerdlow RH, Burns JM, Khan SM (2010) The Alzheimer’s disease mitochondrial cascade hypothesis. J Alzheimer’s Dis : JAD 20(Suppl 2):S265–S279. https://doi.org/10.3233/jad-2010-100339
Moreira PI, Carvalho C, Zhu X, Smith MA, Perry G (2010) Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim Biophys Acta (BBA) - Mol Basis Dis 1802(1):2–10. https://doi.org/10.1016/j.bbadis.2009.10.006
Fan Z, Brooks DJ, Okello A, Edison P (2017) An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 140(3):792–803. https://doi.org/10.1093/brain/aww349
Fan Z, Okello AA, Brooks DJ, Edison P (2015) Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer’s disease. Brain 138(Pt 12):3685–3698. https://doi.org/10.1093/brain/awv288
Van Eldik LJ, Carrillo MC, Cole PE, Feuerbach D, Greenberg BD, Hendrix JA, Kennedy M, Kozauer N et al (2016) The roles of inflammation and immune mechanisms in Alzheimer’s disease. Alzheimer’s Dement: Transl Res Clin Interv 2(2):99–109. https://doi.org/10.1016/j.trci.2016.05.001
Li Q, Liu Y, Sun M (2017) Autophagy and Alzheimer’s disease. Cell Mol Neurobiol 37(3):377–388. https://doi.org/10.1007/s10571-016-0386-8
Zare-shahabadi A, Masliah E, Johnson GVW, Rezaei N (2015) Autophagy in Alzheimer’s disease. Rev Neurosci 26(4):385–395. https://doi.org/10.1515/revneuro-2014-0076
Hong L, Huang HC, Jiang ZF (2014) Relationship between amyloid-beta and the ubiquitin-proteasome system in Alzheimer’s disease. Neurol Res 36(3):276–282. https://doi.org/10.1179/1743132813y.0000000288
Gong B, Radulovic M, Figueiredo-Pereira ME, Cardozo C (2016) The ubiquitin-proteasome system: potential therapeutic targets for Alzheimer’s disease and spinal cord injury. Front Mol Neurosci 9(4). https://doi.org/10.3389/fnmol.2016.00004
Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, Perluigi M, Butterfield DA (2015) Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J Neurochem 133(5):739–749. https://doi.org/10.1111/jnc.13037
Persson T, Popescu BO, Cedazo-Minguez A (2014) Oxidative stress in Alzheimer’s disease: why did antioxidant therapy fail? Oxidative Med Cell Longev 2014:1–11. https://doi.org/10.1155/2014/427318
Sontag J-M, Sontag E (2014) Protein phosphatase 2A dysfunction in Alzheimer’s disease. Front Mol Neurosci 7(16). https://doi.org/10.3389/fnmol.2014.00016
Foley TD, Petro LA, Stredny CM, Coppa TM (2007) Oxidative inhibition of protein phosphatase 2A activity: role of catalytic subunit disulfides. Neurochem Res 32(11):1957–1964. https://doi.org/10.1007/s11064-007-9394-x
Brewer GJ (2014) Alzheimer’s disease causation by copper toxicity and treatment with zinc. Front Aging Neurosci 6:92. https://doi.org/10.3389/fnagi.2014.00092
Watt NT, Whitehouse IJ, Hooper NM (2011) The role of zinc in Alzheimer’s disease. Int J Alzheimers Dis 2011:971021. https://doi.org/10.4061/2011/971021
Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139(Suppl 1):179–197. https://doi.org/10.1111/jnc.13425
Larbi A, Pawelec G, Witkowski JM, Schipper HM, Derhovanessian E, Goldeck D, Fulop T (2009) Dramatic shifts in circulating CD4 but not CD8 T cell subsets in mild Alzheimer’s disease. J Alzheimer’s Dis: JAD 17(1):91–103. https://doi.org/10.3233/jad-2009-1015
Le Page A, Garneau H, Dupuis G, Frost EH, Larbi A, Witkowski JM, Pawelec G, Fulop T (2017) Differential phenotypes of myeloid-derived suppressor and T regulatory cells and cytokine levels in amnestic mild cognitive impairment subjects compared to mild Alzheimer diseased patients. Front Immunol 8:783. https://doi.org/10.3389/fimmu.2017.00783
Sommer A, Winner B, Prots I (2017) The Trojan horse—neuroinflammatory impact of T cells in neurodegenerative diseases. Mol Neurodegener 12:78. https://doi.org/10.1186/s13024-017-0222-8
Swardfager W, Lanctôt K, Rothenburg L, Wong A, Cappell J, Herrmann N (2010) A meta-analysis of cytokines in Alzheimer’s disease. Biol Psychiatry 68(10):930–941. https://doi.org/10.1016/j.biopsych.2010.06.012
Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S et al (2017) Gut microbiome alterations in Alzheimer’s disease. Sci Rep 7(1):13537. https://doi.org/10.1038/s41598-017-13601-y
Zhao Y, Cong L, Jaber V, Lukiw WJ (2017) Microbiome-derived lipopolysaccharide enriched in the perinuclear region of Alzheimer’s disease brain. Front Immunol 8(1064). https://doi.org/10.3389/fimmu.2017.01064
Chen Z, Zhong C (2013) Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol 108:21–43. https://doi.org/10.1016/j.pneurobio.2013.06.004
De Felice FG, Lourenco MV, Ferreira ST (2014) How does brain insulin resistance develop in Alzheimer’s disease? Alzheimers Dement 10(1 Suppl):S26–S32. https://doi.org/10.1016/j.jalz.2013.12.004
Wieser V, Moschen AR, Tilg H (2013) Inflammation, cytokines and insulin resistance: a clinical perspective. Arch Immunol Ther Exp 61(2):119–125. https://doi.org/10.1007/s00005-012-0210-1
Montgomery MK, Turner N (2015) Mitochondrial dysfunction and insulin resistance: an update. Endocrine Connections 4(1):R1–R15. https://doi.org/10.1530/EC-14-0092
Mosconi L, Pupi A, De Leon MJ (2008) Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann N Y Acad Sci 1147:180–195. https://doi.org/10.1196/annals.1427.007
Park K, Gross M, Lee D-H, Holvoet P, Himes JH, Shikany JM, Jacobs DR (2009) Oxidative stress and insulin resistance: the Coronary Artery Risk Development in Young Adults study. Diabetes Care 32(7):1302–1307. https://doi.org/10.2337/dc09-0259
Nikolich-Žugich J (2014) Aging of the T cell compartment in mice and humans: from no naïve expectations to foggy memories. J Immunol (Baltimore, Md : 1950) 193(6):2622–2629. https://doi.org/10.4049/jimmunol.1401174
Head E, Lott IT, Wilcock DM, Lemere CA (2016) Aging in down syndrome and the development of Alzheimer’s disease neuropathology. Curr Alzheimer Res 13(1):18–29
Cole A (2012) GSK3 as a sensor determining cell fate in the brain. Front Mol Neurosci 5(4). https://doi.org/10.3389/fnmol.2012.00004
Martinez-Lopez N, Athonvarangkul D, Singh R (2015) Autophagy and aging. Adv Exp Med Biol 847:73–87. https://doi.org/10.1007/978-1-4939-2404-2_3
Josephs KA, Tosakulwong N, Weigand SD, Murray ME, Whitwell JL, Parisi JE, Dickson DW, Petersen RC (2017) Brain tau deposition linked to systemic causes of death in normal elderly. Neurobiol Aging 50(Supplement C):163–166. https://doi.org/10.1016/j.neurobiolaging.2016.11.011
Pirpamer L, Hofer E, Gesierich B, De Guio F, Freudenberger P, Seiler S, Duering M, Jouvent E et al (2016) Determinants of iron accumulation in the normal aging brain. Neurobiol Aging 43:149–155. https://doi.org/10.1016/j.neurobiolaging.2016.04.002
Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP et al (2017) Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe 21(4):455–466.e454. https://doi.org/10.1016/j.chom.2017.03.002
Lim A, Krajina K, Marsland AL (2013) Peripheral inflammation and cognitive aging. Mod Trends Pharmacopsychiatry 28:175–187. https://doi.org/10.1159/000346362
Gemechu JM, Bentivoglio M (2012) T cell recruitment in the brain during normal aging. Front Cell Neurosci 6:38. https://doi.org/10.3389/fncel.2012.00038
Irizarry MC (2004) Biomarkers of Alzheimer disease in plasma. NeuroRx 1(2):226–234
Holtzman DM, Herz J, Bu G (2012) Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harbor Perspect Med 2(3):a006312. https://doi.org/10.1101/cshperspect.a006312
Percy M, Moalem S, Garcia A, Somerville MJ, Hicks M, Andrews D, Azad A, Schwarz P et al (2008) Involvement of ApoE E4 and H63D in sporadic Alzheimer’s disease in a folate-supplemented Ontario population. J Alzheimer’s Dis: JAD 14(1):69–84
Ali-Rahmani F, Schengrund C-L, Connor JR (2014) HFE gene variants, iron, and lipids: a novel connection in Alzheimer’s disease. Front Pharmacol 5:165. https://doi.org/10.3389/fphar.2014.00165
Guerreiro R, Hardy J (2013) TREM2 and neurodegenerative disease. N Engl J Med 369(16):1569–1570
Zhong L, Chen X-F, Wang T, Wang Z, Liao C, Wang Z, Huang R, Wang D et al (2017) Soluble TREM2 induces inflammatory responses and enhances microglial survival. J Exp Med. https://doi.org/10.1084/jem.20160844
Mastroeni D, Sekar S, Nolz J, Delvaux E, Lunnon K, Mill J, Liang WS, Coleman PD (2017) ANK1 is up-regulated in laser captured microglia in Alzheimer’s brain; the importance of addressing cellular heterogeneity. PLoS One 12(7):e0177814. https://doi.org/10.1371/journal.pone.0177814
Sanchez-Mut JV, Gräff J (2015) Epigenetic alterations in Alzheimer’s disease. Front Behav Neurosci 9:347. https://doi.org/10.3389/fnbeh.2015.00347
Ridge PG, Mukherjee S, Crane PK, Kauwe JS (2013) Alzheimer’s disease: analyzing the missing heritability. PLoS One 8(11):e79771. https://doi.org/10.1371/journal.pone.0079771
Smith AR, Smith RG, Condliffe D, Hannon E, Schalkwyk L, Mill J, Lunnon K (2016) Increased DNA methylation near TREM2 is consistently seen in the superior temporal gyrus in Alzheimer's disease brain. Neurobiol Aging 47:35–40. https://doi.org/10.1016/j.neurobiolaging.2016.07.008
Lunnon K, Smith R, Hannon E, De Jager PL, Srivastava G, Volta M, Troakes C, Al-Sarraj S et al (2014) Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer's disease. Nat Neurosci 17(9):1164–1170. https://doi.org/10.1038/nn.3782
De Jager PL, Srivastava G, Lunnon K, Burgess J, Schalkwyk LC, Yu L, Eaton ML, Keenan BT et al (2014) Alzheimer's disease: early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat Neurosci 17:1156. https://doi.org/10.1038/nn.3786 https://www.nature.com/articles/nn.3786#supplementary-information
Yu C-E, Cudaback E, Foraker J, Thomson Z, Leong L, Lutz F, Gill JA, Saxton A et al (2013) Epigenetic signature and enhancer activity of the human APOE gene. Hum Mol Genet 22(24):5036–5047. https://doi.org/10.1093/hmg/ddt354
Foraker J, Millard SP, Leong L, Thomson Z, Chen S, Keene CD, Bekris LM, Yu CE (2015) The APOE gene is differentially methylated in Alzheimer’s disease. J Alzheimer’s Dis : JAD 48(3):745–755. https://doi.org/10.3233/jad-143060
Celarain N, Sanchez-Ruiz de Gordoa J, Zelaya MV, Roldan M, Larumbe R, Pulido L, Echavarri C, Mendioroz M (2016) TREM2 upregulation correlates with 5-hydroxymethycytosine enrichment in Alzheimer’s disease hippocampus. Clin Epigenetics 8:37. https://doi.org/10.1186/s13148-016-0202-9
Lai KSP, Liu CS, Rau A, Lanctôt KL, Köhler CA, Pakosh M, Carvalho AF, Herrmann N (2017) Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2017-316201
Cunningham C, MacLullich AMJ (2013) At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun 28:1–13. https://doi.org/10.1016/j.bbi.2012.07.012
Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, Culliford D, Perry VH (2009) Systemic inflammation and disease progression in Alzheimer disease. Neurology 73(10):768–774. https://doi.org/10.1212/wnl.0b013e3181b6bb95
Chen B, Soto C, Morales R (2014) Corrigendum to “Peripherally administrated prions reach the brain at sub-infectious quantities in experimental hamsters” [FEBS Lett. 588 (2014) 795-800]. FEBS Lett 588 (17):3308–3309. doi:https://doi.org/10.1016/j.febslet.2014.06.039
Politis A, Olgiati P, Malitas P, Albani D, Signorini A, Polito L, De Mauro S, Zisaki A et al (2010) Vitamin B12 levels in Alzheimer’s disease: association with clinical features and cytokine production. J Alzheimers Dis 19(2):481–488. https://doi.org/10.3233/jad-2010-1252
Holmes C, Cunningham C, Zotova E, Culliford D, Perry VH (2011) Proinflammatory cytokines, sickness behavior, and Alzheimer disease. Neurology 77(3):212–218. https://doi.org/10.1212/wnl.0b013e318225ae07
Jabbari Azad F, Talaei A, Rafatpanah H, Yousefzadeh H, Jafari R, Talaei A, Farid Hosseini R (2014) Association between cytokine production and disease severity in Alzheimer’s disease. Iran J Allergy Asthma Immunol 13(6):433–439
Lovestone S, Simmons A (2010) Combinatorial markers of MCI conversion: cytokines and MRI measures together predict disease progression. Alzheimers Dement 6(4):S62. https://doi.org/10.1016/j.jalz.2010.05.178
Furney SJ, Kronenberg D, Simmons A, Guntert A, Dobson RJ, Proitsi P, Wahlund LO, Kloszewska I et al (2011) Combinatorial markers of mild cognitive impairment conversion to Alzheimer’s disease—cytokines and MRI measures together predict disease progression. J Alzheimer’s Dis: JAD 26(Suppl 3):395–405. https://doi.org/10.3233/jad-2011-0044
Town T, Tan J, Flavell RA, Mullan M (2005) T-cells in Alzheimer’s disease. NeuroMolecular Med 7(3):255–264. https://doi.org/10.1385/nmm:7:3:255
Fehervari Z (2016) Lymphocytes in Alzheimer's disease. Nat Immunol 17:355. https://doi.org/10.1038/ni.3427
Pellicano M, Larbi A, Goldeck D, Colonna-Romano G, Buffa S, Bulati M, Rubino G, Iemolo F et al (2012) Immune profiling of Alzheimer patients. J Neuroimmunol 242(1–2):52–59. https://doi.org/10.1016/j.jneuroim.2011.11.005
Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, Alberoni M, Nemni R, Clerici M (2011) Increased activity of Th-17 and Th-9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer’s disease. Brain Behav Immun 25(3):539–547. https://doi.org/10.1016/j.bbi.2010.12.004
Lueg G, Gross CC, Lohmann H, Johnen A, Kemmling A, Deppe M, Groger J, Minnerup J et al (2015) Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer’s disease. Neurobiol Aging 36(1):81–89. https://doi.org/10.1016/j.neurobiolaging.2014.08.008
Dá Mesquita S, Ferreira AC, Sousa JC, Correia-Neves M, Sousa N, Marques F (2016) Insights on the pathophysiology of Alzheimer’s disease: the crosstalk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci Biobehav Rev 68(supplement C):547–562. https://doi.org/10.1016/j.neubiorev.2016.06.014
Esteras N, Alquezar C, de la Encarnacion A, Martin-Requero A (2016) Lymphocytes in Alzheimer’s disease pathology: altered signaling pathways. Curr Alzheimer Res 13(4):439–449
Ferretti MT, Merlini M, Späni C, Gericke C, Schweizer N, Enzmann G, Engelhardt B, Kulic L et al (2016) T-cell brain infiltration and immature antigen-presenting cells in transgenic models of Alzheimer’s disease-like cerebral amyloidosis. Brain Behav Immun 54(supplement C):211–225. https://doi.org/10.1016/j.bbi.2016.02.009
Busse M, Michler E, von Hoff F, Dobrowolny H, Hartig R, Frodl T, Busse S (2017) Alterations in the peripheral immune system in dementia. Journal of Alzheimer’s disease : JAD 58(4):1303–1313. https://doi.org/10.3233/jad-161304
Bryson KJ, Lynch MA (2016) Linking T cells to Alzheimer’s disease: from neurodegeneration to neurorepair. Curr Opin Pharmacol 26(supplement C):67–73. https://doi.org/10.1016/j.coph.2015.10.003
Maftei M, Thurm F, Schnack C, Tumani H, Otto M, Elbert T, Kolassa I-T, Przybylski M et al (2013) Increased levels of antigen-bound β-amyloid autoantibodies in serum and cerebrospinal fluid of Alzheimer’s disease patients. PLoS One 8(7):e68996. https://doi.org/10.1371/journal.pone.0068996
Sollvander S, Ekholm-Pettersson F, Brundin RM, Westman G, Kilander L, Paulie S, Lannfelt L, Sehlin D (2015) Increased number of plasma B cells producing autoantibodies against Abeta42 protofibrils in Alzheimer’s disease. J Alzheimer’s Dis: JAD 48(1):63–72. https://doi.org/10.3233/jad-150236
Xu W, Kawarabayashi T, Matsubara E, Deguchi K, Murakami T, Harigaya Y, Ikeda M, Amari M et al (2008) Plasma antibodies to Abeta40 and Abeta42 in patients with Alzheimer’s disease and normal controls. Brain Res 1219:169–179. https://doi.org/10.1016/j.brainres.2008.02.060
Paul S, Planque S, Nishiyama Y (2010) Immunological origin and functional properties of catalytic autoantibodies to amyloid beta peptide. J Clin Immunol 30(Suppl 1):S43–S49. https://doi.org/10.1007/s10875-010-9414-5
Taguchi H, Planque S, Nishiyama Y, Szabo P, Weksler ME, Friedland RP, Paul S (2008) Catalytic antibodies to amyloid beta peptide in defense against Alzheimer disease. Autoimmun Rev 7(5):391–397. https://doi.org/10.1016/j.autrev.2008.03.004
Baril L, Nicolas L, Croisile B, Crozier P, Hessler C, Sassolas A, McCormick JB, Trannoy E (2004) Immune response to Abeta-peptides in peripheral blood from patients with Alzheimer’s disease and control subjects. Neurosci Lett 355(3):226–230
Morris G, Berk M, Carvalho AF, Caso JR, Sanz Y, Maes M (2016) The role of microbiota and intestinal permeability in the pathophysiology of autoimmune and neuroimmune processes with an emphasis on inflammatory bowel disease type 1 diabetes and chronic fatigue syndrome. Curr Pharm Des 22(40):6058–6075
Morris G, Berk M, Carvalho A, Caso JR, Sanz Y, Walder K, Maes M (2016) The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0004-2
McAleer JP, Vella AT (2010) Educating CD4 T cells with vaccine adjuvants: lessons from lipopolysaccharide. Trends Immunol 31(11):429–435. https://doi.org/10.1016/j.it.2010.08.005
Xu H, Liew LN, Kuo IC, Huang CH, Goh DL-M, Chua KY (2008) The modulatory effects of lipopolysaccharide-stimulated B cells on differential T-cell polarization. Immunology 125(2):218–228. https://doi.org/10.1111/j.1365-2567.2008.02832.x
Minguet S, Dopfer EP, Pollmer C, Freudenberg MA, Galanos C, Reth M, Huber M, Schamel WW (2008) Enhanced B-cell activation mediated by TLR4 and BCR crosstalk. Eur J Immunol 38(9):2475–2487. https://doi.org/10.1002/eji.200738094
Reynolds JM, Martinez GJ, Chung Y, Dong C (2012) Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci U S A 109(32):13064–13069. https://doi.org/10.1073/pnas.1120585109
Cui W, Joshi NS, Liu Y, Meng H, Kleinstein SH, Kaech SM (2014) TLR4 ligands lipopolysaccharide and monophosphoryl lipid a differentially regulate effector and memory CD8+ T cell differentiation. J Immunol (Baltimore, Md : 1950) 192(9):4221–4232. https://doi.org/10.4049/jimmunol.1302569
McAleer JP, Rossi RJ, Vella AT (2009) Lipopolysaccharide potentiates effector T cell accumulation into non-lymphoid tissues through TRIF. J Immunol (Baltimore, Md : 1950) 182(9):5322–5330. https://doi.org/10.4049/jimmunol.0803616
Komai-Koma M, Gilchrist DS, Xu D (2009) Direct recognition of LPS by human but not murine CD8+ T cells via TLR4 complex. Eur J Immunol 39(6):1564–1572. https://doi.org/10.1002/eji.200838866
Tripathy A, Khanna S, Padhan P, Smita S, Raghav S, Gupta B (2017) Direct recognition of LPS drive TLR4 expressing CD8(+) T cell activation in patients with rheumatoid arthritis. Sci Rep 7(1):933. https://doi.org/10.1038/s41598-017-01033-7
Kanevskiy LM, Telford WG, Sapozhnikov AM, Kovalenko EI (2013) Lipopolysaccharide induces IFN-γ production in human NK cells. Front Immunol 4:11. https://doi.org/10.3389/fimmu.2013.00011
Goodier MR, Londei M (2000) Lipopolysaccharide stimulates the proliferation of human CD56+CD3- NK cells: a regulatory role of monocytes and IL-10. J Immunol (Baltimore, Md : 1950) 165(1):139–147
Masera RG, Prolo P, Sartori ML, Staurenghi A, Griot G, Ravizza L, Dovio A, Chiappelli F et al (2002) Mental deterioration correlates with response of natural killer (NK) cell activity to physiological modifiers in patients with short history of Alzheimer’s disease. Psychoneuroendocrinology 27(4):447–461
Jadidi-Niaragh F, Shegarfi H, Naddafi F, Mirshafiey A (2012) The role of natural killer cells in Alzheimer’s disease. Scand J Immunol 76(5):451–456. https://doi.org/10.1111/j.1365-3083.2012.02769.x
Jofre-Monseny L, Loboda A, Wagner AE, Huebbe P, Boesch-Saadatmandi C, Jozkowicz A, Minihane A-M, Dulak J et al (2007) Effects of apoE genotype on macrophage inflammation and heme oxygenase-1 expression. Biochem Biophys Res Commun 357(1–3):319–324. https://doi.org/10.1016/j.bbrc.2007.03.150
Huebbe P, Lodge JK, Rimbach G (2010) Implications of apolipoprotein E genotype on inflammation and vitamin E status. Mol Nutr Food Res 54(5):623–630. https://doi.org/10.1002/mnfr.200900398
Cash JG, Kuhel DG, Basford JE, Jaeschke A, Chatterjee TK, Weintraub NL, Hui DY (2012) Apolipoprotein E4 impairs macrophage efferocytosis and potentiates apoptosis by accelerating endoplasmic reticulum stress. J Biol Chem 287(33):27876–27884. https://doi.org/10.1074/jbc.M112.377549
Fond AM, Ravichandran KS (2016) Clearance of dying cells by phagocytes: mechanisms and implications for disease pathogenesis. Adv Exp Med Biol 930:25–49. https://doi.org/10.1007/978-3-319-39406-0_2
Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA et al (2008) Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 29(4):637–649. https://doi.org/10.1016/j.immuni.2008.08.009
Ivanov Ivaylo I, Honda K (2012) Intestinal commensal microbes as immune modulators. Cell Host Microbe 12(4):496–508. https://doi.org/10.1016/j.chom.2012.09.009
Atarashi K, Honda K (2008) Analysis of murine lamina propria TH17 cells.
Kumar H, Lund R, Laiho A, Lundelin K, Ley RE, Isolauri E, Salminen S (2014) Gut microbiota as an epigenetic regulator: pilot study based on whole-genome methylation analysis. MBio 5(6). https://doi.org/10.1128/mBio.02113-14
Ye J, Wu W, Li Y, Li L (2017) Influences of the gut microbiota on DNA methylation and histone modification. Dig Dis Sci 62(5):1155–1164. https://doi.org/10.1007/s10620-017-4538-6
Saita D, Ferrarese R, Foglieni C, Esposito A, Canu T, Perani L, Ceresola ER, Visconti L et al (2016) Adaptive immunity against gut microbiota enhances apoE-mediated immune regulation and reduces atherosclerosis and western-diet-related inflammation. Sci Rep 6:29353. https://doi.org/10.1038/srep29353 https://www.nature.com/articles/srep29353#supplementary-information
Song J, Lee JE (2015) miR-155 is involved in Alzheimer’s disease by regulating T lymphocyte function. Frontiers in aging neuroscience 7:61. doi:https://doi.org/10.3389/fnagi.2015.00061
Li H, Guo Z, Guo Y, Li M, Yan H, Cheng J, Wang C, Hong G (2016) Common DNA methylation alterations of Alzheimer’s disease and aging in peripheral whole blood. Oncotarget 7(15):19089–19098. https://doi.org/10.18632/oncotarget.7862
Zhao Y, Bhattacharjee S, Jones BM, Hill J, Dua P, Lukiw WJ (2014) Regulation of neurotropic signaling by the inducible, NF-kB-sensitive miRNA-125b in Alzheimer’s disease (AD) and in primary human neuronal-glial (HNG) cells. Mol Neurobiol 50(1):97–106. https://doi.org/10.1007/s12035-013-8595-3
Yang Y, Wang JK (2016) The functional analysis of microRNAs involved in NF-kappaB signaling. Eur Rev Med Pharmacol Sci 20(9):1764–1774
Naqvi AR, Zhong S, Dang H, Fordham JB, Nares S, Khan A (2016) Expression profiling of LPS responsive miRNA in primary human macrophages. J Microb Biochem Technol 8(2):136–143. https://doi.org/10.4172/1948-5948.1000276
Wang X, Cao Q, Yu L, Shi H, Xue B, Shi H (2016) Epigenetic regulation of macrophage polarization and inflammation by DNA methylation in obesity. JCI Insight 1(19):e87748. https://doi.org/10.1172/jci.insight.87748
Czauderna S, Rudnik M, Losko M, Witalisz A, Jozkowicz A, Dulak J, Loboda A (2015) Influence of apolipoprotein E isoform on microRNA transcriptome in macrophages. Atherosclerosis 241(1):e84. https://doi.org/10.1016/j.atherosclerosis.2015.04.295
Yang X, Wang X, Liu D, Yu L, Xue B, Shi H (2014) Epigenetic regulation of macrophage polarization by DNA methyltransferase 3b. Mol Endocrinol 28(4):565–574. https://doi.org/10.1210/me.2013-1293
Essandoh K, Li Y, Huo J, Fan GC (2016) MiRNA-mediated macrophage polarization and its potential role in the regulation of inflammatory response. Shock (Augusta, Ga) 46(2):122–131. https://doi.org/10.1097/shk.0000000000000604
Mann M, Mehta A, Zhao JL, Lee K, Marinov GK, Garcia-Flores Y, Baltimore D (2017) An NF-κB-microRNA regulatory network tunes macrophage inflammatory responses. Nat Commun 8(1):851. https://doi.org/10.1038/s41467-017-00972-z
Alexandrov PN, Dua P, Lukiw WJ (2014) Up-regulation of miRNA-146a in progressive, age-related inflammatory neurodegenerative disorders of the human CNS. Front Neurol 5:181. https://doi.org/10.3389/fneur.2014.00181
Ito H, Hamerman JA (2012) TREM-2, triggering receptor expressed on myeloid cell-2, negatively regulates TLR responses in dendritic cells. Eur J Immunol 42(1):176–185. https://doi.org/10.1002/eji.201141679
Hall SC, Agrawal DK (2017) Increased TREM-2 expression on the subsets of CD11c+ cells in the lungs and lymph nodes during allergic airway inflammation. Sci Rep 7(1):11853. https://doi.org/10.1038/s41598-017-12330-6
Bailey CC, DeVaux LB, Farzan M (2015) The triggering receptor expressed on myeloid cells 2 binds apolipoprotein E. J Biol Chem 290(43):26033–26042. https://doi.org/10.1074/jbc.M115.677286
Ozaki Y, Yoshino Y, Yamazaki K, Sao T, Mori Y, Ochi S, Yoshida T, Mori T et al (2017) DNA methylation changes at TREM2 intron 1 and TREM2 mRNA expression in patients with Alzheimer’s disease. J Psychiatr Res 92:74–80. https://doi.org/10.1016/j.jpsychires.2017.04.003
Mori Y, Yoshino Y, Ochi S, Yamazaki K, Kawabe K, Abe M, Kitano T, Ozaki Y et al (2015) TREM2 mRNA expression in leukocytes is increased in Alzheimer’s disease and schizophrenia. PLoS One 10(9):e0136835. https://doi.org/10.1371/journal.pone.0136835
Tan YJ, Ng ASL, Vipin A, Lim JKW, Chander RJ, Ji F, Qiu Y, Ting SKS et al (2017) Higher peripheral TREM2 mRNA levels relate to cognitive deficits and hippocampal atrophy in Alzheimer’s disease and amnestic mild cognitive impairment. J Alzheimer’s Dis: JAD 58(2):413–423. https://doi.org/10.3233/jad-161277
Sun L, Pappy Ii AL, Pham TT, Shanley TP (2015) Study of protein phosphatase 2A (PP2A) activity in LPS-induced tolerance using fluorescence-based and immunoprecipitation-aided methodology. Biomol Ther 5(3):1284–1301. https://doi.org/10.3390/biom5031284
Chuang Y-F, Chen M-C, Huang S-W, Hsu Y-F, Ou G, Tsai Y-J, Hsu M-J (2015) Protein phosphatase 2A in lipopolysaccharide-induced cyclooxygenase-2 expression in murine lymphatic endothelial cells. PLoS One 10(8):e0137177. https://doi.org/10.1371/journal.pone.0137177
Morris G, Berk M, Walder K, Maes M (2015) Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med 13(1):28
Morris G, Walker AJ, Berk M, Maes M, Puri BK (2017) Cell death pathways: a novel therapeutic approach for neuroscientists. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0793-y
Streit WJ, Xue Q-S, Tischer J, Bechmann I (2014) Microglial pathology. Acta Neuropathol Commun 2:142. https://doi.org/10.1186/s40478-014-0142-6
Parbo P, Ismail R, Hansen KV, Amidi A, Marup FH, Gottrup H, Braendgaard H, Eriksson BO et al (2017) Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain 140(7):2002–2011. https://doi.org/10.1093/brain/awx120
Solito E, Sastre M (2012) Microglia function in Alzheimer’s disease. Front Pharmacol 3. https://doi.org/10.3389/fphar.2012.00014
Prokop S, Miller KR, Heppner FL (2013) Microglia actions in Alzheimer’s disease. Acta Neuropathol 126(4):461–477. https://doi.org/10.1007/s00401-013-1182-x
Doens D, Fernández PL (2014) Microglia receptors and their implications in the response to amyloid β for Alzheimer’s disease pathogenesis. J Neuroinflammation 11(1):48. https://doi.org/10.1186/1742-2094-11-48
Perry VH, Cunningham C, Holmes C (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7(2):161–167. https://doi.org/10.1038/nri2015
Norden DM, Muccigrosso MM, Godbout JP (2015) Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96((Pt a):29–41. https://doi.org/10.1016/j.neuropharm.2014.10.028
Hoeijmakers L, Heinen Y, van Dam A-M, Lucassen PJ, Korosi A (2016) Microglial priming and Alzheimer’s disease: a possible role for (early) immune challenges and epigenetics? Front Hum Neurosci 10:398. https://doi.org/10.3389/fnhum.2016.00398
Boutajangout A, Wisniewski T (2013) The innate immune system in Alzheimer’s disease. Int J Cell Biol 2013:1–7. https://doi.org/10.1155/2013/576383
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C et al (2013) TREM2 variants in Alzheimer’s disease. N Engl J Med 368(2):117–127. https://doi.org/10.1056/nejmoa1211851
Lue L-F, Schmitz CT, Serrano G, Sue LI, Beach TG, Walker DG (2014) TREM2 protein expression changes correlate with Alzheimer’s disease neurodegenerative pathologies in post-mortem temporal cortices. Brain Pathol 25(4):469–480. https://doi.org/10.1111/bpa.12190
Hickman SE, El Khoury J (2014) TREM2 and the neuroimmunology of Alzheimer’s disease. Biochem Pharmacol 88(4):495–498. https://doi.org/10.1016/j.bcp.2013.11.021
Lue LF, Schmitz C, Walker DG (2015) What happens to microglial TREM2 in Alzheimer’s disease: immunoregulatory turned into immunopathogenic? Neuroscience 302:138–150. https://doi.org/10.1016/j.neuroscience.2014.09.050
Jay TR, von Saucken VE, Landreth GE (2017) TREM2 in neurodegenerative diseases. Mol Neurodegener 12(1):56. https://doi.org/10.1186/s13024-017-0197-5
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, Beckers L, O'Loughlin E et al (2017) The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity 47(3):566–581.e569. https://doi.org/10.1016/j.immuni.2017.08.008
Li X, Montine KS, Keene CD, Montine TJ (2015) Different mechanisms of apolipoprotein E isoform-dependent modulation of prostaglandin E2 production and triggering receptor expressed on myeloid cells 2 (TREM2) expression after innate immune activation of microglia. FASEB J 29(5):1754–1762. https://doi.org/10.1096/fj.14-262683
Zhong L, Chen X-F, Zhang Z-L, Wang Z, Shi X-Z, Xu K, Zhang Y-W, Xu H et al (2015) DAP12 stabilizes the C-terminal fragment of the triggering receptor expressed on myeloid cells-2 (TREM2) and protects against LPS-induced pro-inflammatory response. J Biol Chem 290(25):15866–15877. https://doi.org/10.1074/jbc.M115.645986
Sasaki A, Kakita A, Yoshida K, Konno T, Ikeuchi T, Hayashi S, Matsuo H, Shioda K (2015) Variable expression of microglial DAP12 and TREM2 genes in Nasu-Hakola disease. Neurogenetics 16(4):265–276. https://doi.org/10.1007/s10048-015-0451-3
Zhong L, Zhang ZL, Li X, Liao C, Mou P, Wang T, Wang Z, Wang Z et al (2017) TREM2/DAP12 complex regulates inflammatory responses in microglia via the JNK signaling pathway. Front Aging Neurosci 9:204. https://doi.org/10.3389/fnagi.2017.00204
Kobayashi M, Konishi H, Sayo A, Takai T, Kiyama H (2016) TREM2/DAP12 signal elicits proinflammatory response in microglia and exacerbates neuropathic pain. J Neurosci 36(43):11138–11150. https://doi.org/10.1523/jneurosci.1238-16.2016
Wunderlich P, Glebov K, Kemmerling N, Tien NT, Neumann H, Walter J (2013) Sequential proteolytic processing of the triggering receptor expressed on myeloid Cells-2 (TREM2) protein by ectodomain shedding and γ-secretase-dependent intramembranous cleavage. J Biol Chem 288(46):33027–33036. https://doi.org/10.1074/jbc.M113.517540
Klein H-U, Bennett DA, De Jager PL (2016) The epigenome in Alzheimer’s disease: current state and approaches for a new path to gene discovery and understanding disease mechanism. Acta Neuropathol 132(4):503–514. https://doi.org/10.1007/s00401-016-1612-7
Hall AE, Lu WT, Godfrey JD, Antonov AV, Paicu C, Moxon S, Dalmay T, Wilczynska A et al (2016) The cytoskeleton adaptor protein ankyrin-1 is upregulated by p53 following DNA damage and alters cell migration. Cell Death Dis 7(4):e2184. https://doi.org/10.1038/cddis.2016.91
Lulli V, Romania P, Morsilli O, Cianciulli P, Gabbianelli M, Testa U, Giuliani A, Marziali G (2013) MicroRNA-486-3p regulates gamma-globin expression in human erythroid cells by directly modulating BCL11A. PLoS One 8(4):e60436. https://doi.org/10.1371/journal.pone.0060436
Tessema M, Yingling CM, Picchi MA, Wu G, Ryba T, Lin Y, Bungum AO, Edell ES et al (2017) ANK1 methylation regulates expression of microRNA-486-5p and discriminates lung tumors by histology and smoking status. Cancer Lett 410:191–200. https://doi.org/10.1016/j.canlet.2017.09.038
Caserta S, Kern F, Cohen J, Drage S, Newbury SF, Llewelyn MJ (2016) Circulating plasma microRNAs can differentiate human sepsis and systemic inflammatory response syndrome (SIRS). Sci Rep 6:28006. https://doi.org/10.1038/srep28006
Zhu J, Zeng Y, Xu C, Qin H, Lei Z, Shen D, Liu Z, Huang JA (2015) Expression profile analysis of microRNAs and downregulated miR-486-5p and miR-30a-5p in non-small cell lung cancer. Oncol Rep 34(4):1779–1786. https://doi.org/10.3892/or.2015.4141
Youness RA, El-Tayebi HM, Assal RA, Hosny K, Esmat G, Abdelaziz AI (2016) MicroRNA-486-5p enhances hepatocellular carcinoma tumor suppression through repression of IGF-1R and its downstream mTOR, STAT3 and c-Myc. Oncol Lett 12(4):2567–2573. https://doi.org/10.3892/ol.2016.4914
Xu Y, Wang Y, Yao A, Xu Z, Dou H, Shen S, Hou Y, Wang T (2017) Low frequency magnetic fields induce autophagy-associated cell death in lung cancer through miR-486-mediated inhibition of Akt/mTOR signaling pathway. Sci Rep 7(1):11776. https://doi.org/10.1038/s41598-017-10407-w
Dello Russo C, Lisi L, Tringali G, Navarra P (2009) Involvement of mTOR kinase in cytokine-dependent microglial activation and cell proliferation. Biochem Pharmacol 78(9):1242–1251. https://doi.org/10.1016/j.bcp.2009.06.097
Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C (2017) Akt signaling pathway in macrophage activation and M1/M2 polarization. J Immunol (Baltimore, Md : 1950) 198(3):1006–1014. https://doi.org/10.4049/jimmunol.1601515
Lu Y, He M, Zhang Y, Xu S, Zhang L, He Y, Chen C, Liu C et al (2014) Differential pro-inflammatory responses of astrocytes and microglia involve STAT3 activation in response to 1800 MHz radiofrequency fields. PLoS One 9(9):e108318. https://doi.org/10.1371/journal.pone.0108318
Guillot-Sestier MV, Doty KR, Gate D, Rodriguez J Jr, Leung BP, Rezai-Zadeh K, Town T (2015) Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron 85(3):534–548. https://doi.org/10.1016/j.neuron.2014.12.068
Doty KR, Guillot-Sestier M-V, Town T Stat3 signaling referees microglial amyloid clearance in Alzheimer’s disease. Alzheimer’s Dement: J Alzheimer’s Assoc 12(7):P1150. https://doi.org/10.1016/j.jalz.2016.07.047
Ulland TK, Song WM, Huang SC-C, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y et al TREM2 maintains microglial metabolic fitness in Alzheimer’s disease. Cell 170(4):649–663.e613. https://doi.org/10.1016/j.cell.2017.07.023
Li X, Long J, He T, Belshaw R, Scott J (2015) Integrated genomic approaches identify major pathways and upstream regulators in late onset Alzheimer’s disease. Sci Rep 5:12393. https://doi.org/10.1038/srep12393
Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, Zhang C, Xie T et al (2013) Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153(3):707–720. https://doi.org/10.1016/j.cell.2013.03.030
Zuroff L, Daley D, Black KL, Koronyo-Hamaoui M (2017) Clearance of cerebral Aβ in Alzheimer’s disease: reassessing the role of microglia and monocytes. Cell Mol Life Sci 74(12):2167–2201. https://doi.org/10.1007/s00018-017-2463-7
Liu S, Liu Y, Hao W, Wolf L, Kiliaan AJ, Penke B, Rube CE, Walter J et al (2011) TLR2 is a primary receptor for Alzheimer’s amyloid peptide to trigger neuroinflammatory activation. J Immunol 188(3):1098–1107. https://doi.org/10.4049/jimmunol.1101121
Streit WJ, Braak H, Xue QS, Bechmann I (2009) Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer’s disease. Acta Neuropathol 118(4):475–485. https://doi.org/10.1007/s00401-009-0556-6
Verkhratsky A, Olabarria M, Noristani HN, Yeh C-Y, Rodriguez JJ (2010) Astrocytes in Alzheimer’s disease. Neurotherapeutics : J Am Soc Exp Neuro Ther 7(4):399–412. https://doi.org/10.1016/j.nurt.2010.05.017
Rodríguez JJ, Olabarria M, Chvatal A, Verkhratsky A (2008) Astroglia in dementia and Alzheimer’s disease. Cell Death Differ 16(3):378–385. https://doi.org/10.1038/cdd.2008.172
Gee JR, Keller JN (2005) Astrocytes: regulation of brain homeostasis via apolipoprotein E. Int J Biochem Cell Biol 37(6):1145–1150. https://doi.org/10.1016/j.biocel.2004.10.004
Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, Betsholtz C et al (2012) Apolipoprotein E controls cerebrovascular integrity via cyclophilin a. Nature. https://doi.org/10.1038/nature11087
Mahley RW, Weisgraber KH, Huang Y (2006) Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci 103(15):5644–5651. https://doi.org/10.1073/pnas.0600549103
Lasagna-Reeves CA, Kayed R (2011) Astrocytes contain amyloid-β annular protofibrils in Alzheimer’s disease brains. FEBS Lett 585(19):3052–3057. https://doi.org/10.1016/j.febslet.2011.08.027
Zhao J, O'Connor T, Vassar R (2011) The contribution of activated astrocytes to Aβ production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation 8(1):150. https://doi.org/10.1186/1742-2094-8-150
Dal Prà I, Chiarini A, Pacchiana R, Gardenal E, Chakravarthy B, Whitfield JF, Armato U (2014) Calcium-sensing receptors of human astrocyte-neuron teams: amyloid-β-driven mediators and therapeutic targets of Alzheimer’s disease. Curr Neuropharmacol 12(4):353–364. https://doi.org/10.2174/1570159X12666140828214701
Vaziri ND (2008) Causal link between oxidative stress, inflammation, and hypertension. Iran J Kidney Dis 2(1):1–10
Lucas K, Morris G, Anderson G, Maes M (2015) The toll-like receptor radical cycle pathway: a new drug target in immune-related chronic fatigue. CNS Neurol Disord Drug Targets 14(7):838–854
Jomova K, Vondrakova D, Lawson M, Valko M (2010) Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 345(1–2):91–104. https://doi.org/10.1007/s11010-010-0563-x
Chang S, Ma T, Miranda RD, Balestra ME, Mahley RW, Huang Y (2005) Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci 102(51):18694–18699. https://doi.org/10.1073/pnas.0508254102
Markesbery WR, Lovell MA (2007) Damage to lipids, proteins, DNA, and RNA in mild cognitive impairment. Arch Neurol 64(7):954. https://doi.org/10.1001/archneur.64.7.954
Ding Q, Dimayuga E, Keller J (2007) Oxidative damage, protein synthesis, and protein degradation in Alzheimers disease. Curr Alzheimer Res 4(1):73–79. https://doi.org/10.2174/156720507779939788
Chen L, Na R, Gu M, Richardson A, Ran Q (2008) Lipid peroxidation up-regulates BACE1 expression in vivo: a possible early event of amyloidogenesis in Alzheimer’s disease. J Neurochem 107(1):197–207. https://doi.org/10.1111/j.1471-4159.2008.05603.x
Quiroz-Baez R, Rojas E, Arias C (2009) Oxidative stress promotes JNK-dependent amyloidogenic processing of normally expressed human APP by differential modification of α-, β- and γ-secretase expression. Neurochem Int 55(7):662–670. https://doi.org/10.1016/j.neuint.2009.06.012
Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo D-G, Mattson MP, Tabaton M (2007) Oxidative stress activates a positive feedback between the γ- and β-secretase cleavages of the β-amyloid precursor protein. Journal of neurochemistry 0 (0):071115163316002-??? doi:https://doi.org/10.1111/j.1471-4159.2007.05072.x
Shen C, Chen Y, Liu H, Zhang K, Zhang T, Lin A, Jing N (2008) Hydrogen peroxide promotes Aβ production through JNK-dependent activation of γ-secretase. J Biol Chem 283(25):17721–17730. https://doi.org/10.1074/jbc.M800013200
Sambamurti K (2004) Gene structure and organization of the human -secretase (BACE) promoter. FASEB J. https://doi.org/10.1096/fj.03-1378fje
Matsui T, Ingelsson M, Fukumoto H, Ramasamy K, Kowa H, Frosch MP, Irizarry MC, Hyman BT (2007) Expression of APP pathway mRNAs and proteins in Alzheimer’s disease. Brain Res 1161:116–123. https://doi.org/10.1016/j.brainres.2007.05.050
Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L et al (2003) Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med 9(1):3–4. https://doi.org/10.1038/nm0103-3
Yao M (2005) Amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J Neurosci 25(5):1149–1158. https://doi.org/10.1523/jneurosci.4736-04.2005
Alavi Naini SM, Soussi-Yanicostas N (2015) Tau hyperphosphorylation and oxidative stress, a critical vicious circle in neurodegenerative Tauopathies? Oxidative Med Cell Longev 2015:17. https://doi.org/10.1155/2015/151979
Liu Z, Li T, Li P, Wei N, Zhao Z, Liang H, Ji X, Chen W et al (2015) The ambiguous relationship of oxidative stress, tau hyperphosphorylation, and autophagy dysfunction in Alzheimer’s disease. Oxidative Med Cell Longev 2015:352723. https://doi.org/10.1155/2015/352723
Giraldo E, Lloret A, Fuchsberger T, Viña J (2014) Aβ and tau toxicities in Alzheimer’s are linked via oxidative stress-induced p38 activation: protective role of vitamin E. Redox biology 2(supplement C):873–877. https://doi.org/10.1016/j.redox.2014.03.002
Gamblin TC, King ME, Kuret J, Berry RW, Binder LI (2000) Oxidative regulation of fatty acid-induced tau polymerization†. Biochemistry 39(46):14203–14210. https://doi.org/10.1021/bi001876l
Zhu X, Raina AK, Rottkamp CA, Boux H, Siedlak SL, Takeda A, Perry G, Smith MA (2000) Activation of P38 kinase links π phosphorylation, oxidative stress and cell cycle-related events in Alzheimer disease. Neurobiol Aging 21:267. https://doi.org/10.1016/s0197-4580(00)83154-1
Praticò D (1999) F2-isoprostanes: sensitive and specific non-invasive indices of lipid peroxidation in vivo. Atherosclerosis 147(1):1–10. https://doi.org/10.1016/s0021-9150(99)00257-9
Ramassamy C, Averill D, Beffert U, Theroux L, Lussier-Cacan S, Cohn JS, Christen Y, Schoofs A et al (2000) Oxidative insults are associated with apolipoprotein E genotype in Alzheimer’s disease brain. Neurobiol Dis 7(1):23–37. https://doi.org/10.1006/nbdi.1999.0273
Shea TB, Rogers E, Ashline D, Ortiz D, Sheu M-S (2002) Apolipoprotein E deficiency promotes increased oxidative stress and compensatory increases in antioxidants in brain tissue. Free Radic Biol Med 33(8):1115–1120. https://doi.org/10.1016/s0891-5849(02)01001-8
Dorszewska J, Polrolniczak A, Prendecki M, Florczak J, Dezor M, Kowalska M, Postrach I, Kozubski W (2014) Apolipoprotein E genotype and oxidative stress in peripheral lymphocytes of patients with Alzheimer’s disease. Alzheimers Dement 10(4):P674. https://doi.org/10.1016/j.jalz.2014.05.1213
Ossenkoppele R, Schonhaut DR, Schöll M, Lockhart SN, Ayakta N, Baker SL, O’Neil JP, Janabi M et al (2016) Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain 139(5):1551–1567. https://doi.org/10.1093/brain/aww027
Moreira PI, Cardoso SM, Santos MS, Oliveira CR (2006) The key role of mitochondria in Alzheimer’s disease. J Alzheimers Dis 9(2):101–110. https://doi.org/10.3233/jad-2006-9202
Caspersen C (2005) Mitochondrial a : a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. https://doi.org/10.1096/fj.05-3735fje
Wang X, Wang W, Li L, Perry G, Lee H-g, Zhu X (2014) Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta (BBA) - Mol Basis Dis 1842(8):1240–1247. https://doi.org/10.1016/j.bbadis.2013.10.015
Zhu X, Perry G, Moreira PI, Aliev G, Cash AD, Hirai K, Smith MA (2006) Mitochondrial abnormalities and oxidative imbalance in Alzheimer disease. J Alzheimers Dis 9(2):147–153. https://doi.org/10.3233/jad-2006-9207
Onyango IG, Dennis J, Khan SM (2016) Mitochondrial dysfunction in Alzheimer’s disease and the rationale for bioenergetics based therapies. Aging Dis (7, 2):201–214. https://doi.org/10.14336/AD.2015.1007
Eckert A, Schmitt K, Götz J (2011) Mitochondrial dysfunction—the beginning of the end in Alzheimer’s disease? Separate and synergistic modes of tau and amyloid-β toxicity. Alzheimers Res Ther 3(2):15. https://doi.org/10.1186/alzrt74
Mancuso M, Orsucci D, LoGerfo A, Calsolaro V, Siciliano G (2010) Clinical features and pathogenesis of Alzheimer’s disease: Involvement of mitochondria and mitochondrial DNA. Adv Exp Med Biol Springer New York. https://doi.org/10.1007/978-1-4419-6448-9_4
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8(2):101–112. https://doi.org/10.1038/nrm2101
Morris JK, Honea RA, Vidoni ED, Swerdlow RH, Burns JM (2014) Is Alzheimer’s disease a systemic disease? Biochim Biophys Acta (BBA) - Mol Basis Dis 1842(9):1340–1349. https://doi.org/10.1016/j.bbadis.2014.04.012
Lunnon K, Ibrahim Z, Proitsi P, Lourdusamy A, Newhouse S, Sattlecker M, Furney S, Saleem M et al (2012) Mitochondrial dysfunction and immune activation are detectable in early Alzheimer’s disease blood. J Alzheimer’s Dis: JAD 30(3):685–710. https://doi.org/10.3233/jad-2012-111592
Leuner K, Schulz K, Schütt T, Pantel J, Prvulovic D, Rhein V, Savaskan E, Czech C et al (2012) Peripheral mitochondrial dysfunction in Alzheimer’s disease: focus on lymphocytes. Mol Neurobiol 46(1):194–204. https://doi.org/10.1007/s12035-012-8300-y
Morris G, Berk M, Klein H, Walder K, Galecki P, Maes M (2016) Nitrosative stress, hypernitrosylation, and autoimmune responses to nitrosylated proteins: new pathways in neuroprogressive disorders including depression and chronic fatigue syndrome. Mol Neurobiol. https://doi.org/10.1007/s12035-016-9975-2
Morris G, Maes M (2014) Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr Neuropharmacol 12(2):168–185. https://doi.org/10.2174/1570159x11666131120224653
Morris G, Maes M (2014) Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab Brain Dis 29:19–36
Morris G, Walder K, Carvalho AF, Tye SJ, Lucas K, Berk M, Maes M (2017) The role of hypernitrosylation in the pathogenesis and pathophysiology of neuroprogressive diseases. Neurosci Biobehav Rev. https://doi.org/10.1016/j.neubiorev.2017.07.017
Gibson GE, Sheu KFR, Blass JP (1998) Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm 105(8–9):855–870. https://doi.org/10.1007/s007020050099
Orsucci D, Mancuso M, Caldarazzo Ienco E, Simoncini C, Siciliano G, Bonuccelli U (2012) Vascular factors and mitochondrial dysfunction: a central role in the pathogenesis of Alzheimer’s disease. Curr Neurovasc Res 10(1):76–80. https://doi.org/10.2174/1567202611310010010
Mise A, Yoshino Y, Yamazaki K, Ozaki Y, Sao T, Yoshida T, Mori T, Mori Y et al (2017) TOMM40 and APOE gene expression and cognitive decline in Japanese Alzheimer’s disease subjects. J Alzheimer’s Dis: JAD 60(3):1107–1117. https://doi.org/10.3233/jad-170361
Huang H, Zhao J, Xu B, Ma X, Dai Q, Li T, Xue F, Chen B (2016) The TOMM40 gene rs2075650 polymorphism contributes to Alzheimer’s disease in Caucasian, and Asian populations. Neurosci Lett 628:142–146. https://doi.org/10.1016/j.neulet.2016.05.050
Gottschalk WK, Lutz MW, He YT, Saunders AM, Burns DK, Roses AD, Chiba-Falek O (2014) The broad impact of TOM40 on neurodegenerative diseases in aging. J Parkinson’s Dis Alzheimer’s Dis 1(1):12. https://doi.org/10.13188/2376-922X.1000003
Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N (2016) Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol 53(1):648–661. https://doi.org/10.1007/s12035-014-9053-6
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimers Dis 57(4):1105–1121. https://doi.org/10.3233/JAD-161088
Valla J, Yaari R, Wolf AB, Kusne Y, Beach TG, Roher AE, Corneveaux JJ, Huentelman MJ et al (2010) Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimer’s Dis: JAD 22(1):307–313. https://doi.org/10.3233/jad-2010-100129
Knopman DS, Jack CR Jr, Wiste HJ, Lundt ES, Weigand SD, Vemuri P, Lowe VJ, Kantarci K et al (2014) 18F-fluorodeoxyglucose positron emission tomography, aging, and apolipoprotein E genotype in cognitively normal persons. Neurobiol Aging 35(9):2096–2106. https://doi.org/10.1016/j.neurobiolaging.2014.03.006
Guo L, Tian J, Du H (2017) Mitochondrial dysfunction and synaptic transmission failure in Alzheimer’s disease. J Alzheimer’s Dis: JAD 57(4):1071–1086. https://doi.org/10.3233/JAD-160702
Tangvarasittichai S (2015) Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes 6(3):456–480. https://doi.org/10.4239/wjd.v6.i3.456
Castellano CA, Baillargeon JP, Nugent S, Tremblay S, Fortier M, Imbeault H, Duval J, Cunnane SC (2015) Regional brain glucose hypometabolism in young women with polycystic ovary syndrome: possible link to mild insulin resistance. PLoS One 10(12):e0144116. https://doi.org/10.1371/journal.pone.0144116
Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S (2011) Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 68(1):51–57. https://doi.org/10.1001/archneurol.2010.225
Vos M, Lauwers E, Verstreken P (2010) Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci 2:139. https://doi.org/10.3389/fnsyn.2010.00139
Du H, Guo L, Yan SS (2012) Synaptic mitochondrial pathology in Alzheimer’s disease. Antioxid Redox Signal 16(12):1467–1475. https://doi.org/10.1089/ars.2011.4277
Cai Q, Tammineni P (2017) Mitochondrial aspects of synaptic dysfunction in Alzheimer’s disease. J Alzheimer’s Dis: JAD 57(4):1087–1103. https://doi.org/10.3233/JAD-160726
Morris G, Maes M (2014) Oxidative and nitrosative stress and immune-inflammatory pathways in patients with myalgic encephalomyelitis (ME)/chronic fatigue syndrome (CFS). Curr Neuropharmacol 12:168–185
Morris G, Walder K, Puri BK, Berk M, Maes M (2016) The deleterious effects of oxidative and nitrosative stress on palmitoylation, membrane lipid rafts and lipid-based cellular signalling: new drug targets in neuroimmune disorders. Mol Neurobiol 53(7):4638–4658. https://doi.org/10.1007/s12035-015-9392-y
Arai M, Saito M, Takatsu H, Fukui K, Urano S (2011) Dysfunction of the fusion of pre-synaptic plasma membranes and synaptic vesicles caused by oxidative stress, and its prevention by vitamin E. J Alzheimer’s Dis: JAD 24(4):759–766. https://doi.org/10.3233/jad-2011-101785
Omoi NO, Arai M, Saito M, Takatsu H, Shibata A, Fukuzawa K, Sato K, Abe K et al (2006) Influence of oxidative stress on fusion of pre-synaptic plasma membranes of the rat brain with phosphatidyl choline liposomes, and protective effect of vitamin E. J Nutr Sci Vitaminol 52(4):248–255
Wang X, Michaelis E (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2(12). https://doi.org/10.3389/fnagi.2010.00012
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol 14(1):101–115. https://doi.org/10.2174/1570159X13666150716165726
A. G S, Jane R (2011) Impact of oxidative - nitrosative stress on cholinergic presynaptic function. Alzheimer’s Disease Pathogenesis-Core Concepts, Shifting Paradigms and Therapeutic Targets. InTech. doi:https://doi.org/10.5772/20198
Rodríguez-Fuentes G, Rubio-Escalante FJ, Noreña-Barroso E, Escalante-Herrera KS, Schlenk D (2015) Impacts of oxidative stress on acetylcholinesterase transcription, and activity in embryos of zebrafish (Danio rerio) following chlorpyrifos exposure. Comp Biochem Physiol Part C: Toxicol Pharmacol 172-173:19–25. https://doi.org/10.1016/j.cbpc.2015.04.003
Santi A, Menezes C, Duarte MMF, Leitemperger J, Lópes T, Loro VL (2011) Oxidative stress biomarkers and acetylcholinesterase activity in human erythrocytes exposed to clomazone (in vitro). Interdiscip Toxicol 4(3):149–153. https://doi.org/10.2478/v10102-011-0023-9
Chen X, Guo C, Kong J (2012) Oxidative stress in neurodegenerative diseases. Neural Regen Res 7(5):376–385. https://doi.org/10.3969/j.issn.1673-5374.2012.05.009
Fu Q, Gao N, Yu J, Ma G, Du Y, Wang F, Su Q, Che F (2014) Diazoxide pretreatment prevents Abeta1-42 induced oxidative stress in cholinergic neurons via alleviating NOX2 expression. Neurochem Res 39(7):1313–1321. https://doi.org/10.1007/s11064-014-1313-3
Zhang Y, Li P, Feng J, Wu M (2016) Dysfunction of NMDA receptors in Alzheimer's disease. Neurol Sci: Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 37(7):1039–1047. https://doi.org/10.1007/s10072-016-2546-5
Steullet P, Neijt HC, Cuenod M, Do KQ (2006) Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: Relevance to schizophrenia. Neuroscience 137(3):807–819. https://doi.org/10.1016/j.neuroscience.2005.10.014
Morris G, Berk M (2015) The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med 13(1):68
Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J (2004) Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A 101(1):284–289. https://doi.org/10.1073/pnas.2635903100
Iaccarino L, Tammewar G, Ayakta N, Baker SL, Bejanin A, Boxer AL, Gorno-Tempini ML, Janabi M et al (2018) Local and distant relationships between amyloid, tau and neurodegeneration in Alzheimer’s disease. NeuroImage : Clinical 17:452–464. https://doi.org/10.1016/j.nicl.2017.09.016
Kang JM, Lee S-Y, Seo S, Jeong HJ, Woo S-H, Lee H, Lee Y-B, Yeon BK et al (2017) Tau positron emission tomography using [18F]THK5351 and cerebral glucose hypometabolism in Alzheimer’s disease. Neurobiology of aging 59(supplement C):210–219. https://doi.org/10.1016/j.neurobiolaging.2017.08.008
Chiotis K, Saint-Aubert L, Rodriguez-Vieitez E, Leuzy A, Almkvist O, Savitcheva I, Jonasson M, Lubberink M et al (2017) Longitudinal changes of tau PET imaging in relation to hypometabolism in prodromal and Alzheimer’s disease dementia. Mol Psychiatry. https://doi.org/10.1038/mp.2017.108 https://www.nature.com/articles/mp2017108#supplementary-information
Bischof GN, Jessen F, Fliessbach K, Dronse J, Hammes J, Neumaier B, Onur O, Fink GR et al (2016) Impact of tau and amyloid burden on glucose metabolism in Alzheimer’s disease. Ann Clin Transl Neurol 3(12):934–939. https://doi.org/10.1002/acn3.339
Kanninen K, White AR, Koistinaho J, Malm T (2011) Targeting glycogen synthase kinase-3β for therapeutic benefit against oxidative stress in Alzheimer’s disease: involvement of the Nrf2-ARE pathway. International Journal of Alzheimer’s Disease 2011:9. https://doi.org/10.4061/2011/985085
Cai Z, Zhao Y, Zhao B (2012) Roles of glycogen synthase kinase 3 in Alzheimer’s disease. Curr Alzheimer Res 9(7):864–879
Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, Berk M (2017) A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav rev 74(Pt a):1–20. https://doi.org/10.1016/j.neubiorev.2017.01.014
Bradley C, Peineau S, Taghibiglou C, Nicolas C, Whitcomb D, Bortolotto Z, Kaang B-K, Cho K et al (2012) A pivotal role of GSK-3 in synaptic plasticity. Front Mol Neurosci 5(13). https://doi.org/10.3389/fnmol.2012.00013
Hooper C, Killick R, Lovestone S (2008) The GSK3 hypothesis of Alzheimer’s disease. J Neurochem 104(6):1433–1439. https://doi.org/10.1111/j.1471-4159.2007.05194.x
Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW (2004) Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci 101(7):2173–2178. https://doi.org/10.1073/pnas.0308512100
Leroy K, Yilmaz Z, Brion JP (2007) Increased level of active GSK-3? In Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol Appl Neurobiol 33(1). https://doi.org/10.1111/j.1365-2990.2006.00795.x
Pei J-J, Tanaka T, Tung Y-C, Braak EVA, Iqbal K, Grundke-Iqbal I (1997) Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J Neuropathol Exp Neurol 56(1):70–78. https://doi.org/10.1097/00005072-199701000-00007
Pei J-J, Braak E, Braak H, Grundke-Iqbal I, Iqbal K, Winblad B, Cowburn RF (1999) Distribution of active glycogen synthase kinase 3β (GSK-3β) in brains staged for Alzheimer disease neurofibrillary changes. J Neuropathol Exp Neurol 58(9):1010–1019. https://doi.org/10.1097/00005072-199909000-00011
Yamaguchi H, Ishiguro K, Uchida T, Takashima A, Lemere CA, Imahori K (1996) Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3β and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathol 92(3):232–241. https://doi.org/10.1007/s004010050513
Hye A, Kerr F, Archer N, Foy C, Poppe M, Brown R, Hamilton G, Powell J et al (2004) Glycogen synthase kinase-3 is increased in white cells early in Alzheimer’s disease. Neurosci Lett 373(1):1–4. https://doi.org/10.1016/j.neulet.2004.10.031
Mateo I, Infante J, Llorca J, Rodríguez E, Berciano J, Combarros O (2006) Association between glycogen synthase kinase-3β genetic polymorphism and late-onset Alzheimer’s disease. Dement Geriatr Cogn Disord 21(4):228–232. https://doi.org/10.1159/000091044
Asuni AA, Hooper C, Reynolds CH, Lovestone S, Anderton BH, Killick R (2006) GSK3α exhibits β-catenin and tau directed kinase activities that are modulated by Wnt. Eur J Neurosci 24(12):3387–3392. https://doi.org/10.1111/j.1460-9568.2006.05243.x
Cho JH, Johnson GV (2004) Primed phosphorylation of tau at Thr231 by glycogen synthase kinase 3beta (GSK3beta) plays a critical role in regulating tau's ability to bind and stabilize microtubules. J Neurochem 88(2):349–358
Sun X, Sato S, Murayama O, Murayama M, Park JM, Yamaguchi H, Takashima A (2002) Lithium inhibits amyloid secretion in COS7 cells transfected with amyloid precursor protein C100. Neurosci Lett 321(1–2):61–64. https://doi.org/10.1016/s0304-3940(01)02583-6
Phiel CJ, Wilson CA, Lee VMY, Klein PS (2003) GSK-3α regulates production of Alzheimer’s disease amyloid-β peptides. Nature 423(6938):435–439. https://doi.org/10.1038/nature01640
Sudduth TL, Schmitt FA, Nelson PT, Wilcock DM (2013) Neuroinflammatory phenotype in early Alzheimer’s disease. Neurobiol Aging 34(4):1051–1059. https://doi.org/10.1016/j.neurobiolaging.2012.09.012
Revesz T, Holton JL, Lashley T, Plant G, Rostagno A, Ghiso J, Frangione B (2006) Sporadic and familial cerebral amyloid angiopathies. Brain Pathol 12(3):343–357. https://doi.org/10.1111/j.1750-3639.2002.tb00449.x
Zhang H, Ma Q, Zhang YW, Xu H (2012) Proteolytic processing of Alzheimer’s beta-amyloid precursor protein. J Neurochem 120(Suppl 1):9–21. https://doi.org/10.1111/j.1471-4159.2011.07519.x
He YJ, Wei PR, Wu QY, Zhang XY, Zhang XM, Liu XJ, Wang F (2016) ApoE4 increases glycogen synthase kinase 3beta expression and tau phosphorylation in U87 cells. Nan fang yi ke da xue xue bao = Journal of Southern Medical University 36(7):904–908
Cedazo-Minguez A, Popescu BO, Blanco-Millan JM, Akterin S, Pei JJ, Winblad B, Cowburn RF (2003) Apolipoprotein E and beta-amyloid (1-42) regulation of glycogen synthase kinase-3beta. J Neurochem 87(5):1152–1164
Markaki M, Tavernarakis N (2013) Metabolic control by target of rapamycin and autophagy during ageing—a mini-review. Gerontology 59(4):340–348
Yu L, Chen Y, Tooze SA (2017) Autophagy pathway: cellular and molecular mechanisms. Autophagy:1–9. https://doi.org/10.1080/15548627.2017.1378838
Oddo S (2008) The ubiquitin-proteasome system in Alzheimer’s disease. J Cell Mol Med 12(2):363–373. https://doi.org/10.1111/j.1582-4934.2008.00276.x
Guglielmotto M, Monteleone D, Vasciaveo V, Repetto IE, Manassero G, Tabaton M, Tamagno E (2017) The decrease of Uch-L1 activity is a common mechanism responsible for Aβ 42 accumulation in Alzheimer’s and vascular disease. Front Aging Neurosci 9:320. https://doi.org/10.3389/fnagi.2017.00320
Orr ME, Oddo S (2013) Autophagic/lysosomal dysfunction in Alzheimer’s disease. Alzheimers Res Ther 5(5):53. https://doi.org/10.1186/alzrt217
Lipinski MM, Zheng B, Lu T, Yan Z, Py BF, Ng A, Xavier RJ, Li C et al (2010) Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A 107(32):14164–14169. https://doi.org/10.1073/pnas.1009485107
Ginsberg SD, Mufson EJ, Counts SE, Wuu J, Alldred MJ, Nixon RA, Che S (2010) Regional selectivity of rab5 and rab7 protein upregulation in mild cognitive impairment and Alzheimer’s disease. J Alzheimer’s Dis: JAD 22(2):631–639. https://doi.org/10.3233/jad-2010-101080
Mishto M, Bellavista E, Santoro A, Stolzing A, Ligorio C, Nacmias B, Spazzafumo L, Chiappelli M et al (2006) Immunoproteasome and LMP2 polymorphism in aged and Alzheimer’s disease brains. Neurobiol Aging 27(1):54–66. https://doi.org/10.1016/j.neurobiolaging.2004.12.004
Choi J, Levey AI, Weintraub ST, Rees HD, Gearing M, Chin LS, Li L (2004) Oxidative modifications and down-regulation of ubiquitin carboxyl-terminal hydrolase L1 associated with idiopathic Parkinson’s and Alzheimer’s diseases. J Biol Chem 279(13):13256–13264. https://doi.org/10.1074/jbc.M314124200
Hands SL, Proud CG, Wyttenbach A (2009) mTOR’s role in ageing: protein synthesis or autophagy? Aging 1(7):586–597
Laplante M, Sabatini DM (2009) mTOR signaling at a glance. J Cell Sci 122(Pt 20):3589–3594. https://doi.org/10.1242/jcs.051011
Laplante M, Sabatini David M (2012) mTOR signaling in growth control and disease. Cell 149(2):274–293. https://doi.org/10.1016/j.cell.2012.03.017
Maiese K (2014) Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med 46(8):587–596. https://doi.org/10.3109/07853890.2014.941921
Sun YX, Ji X, Mao X, Xie L, Jia J, Galvan V, Greenberg DA, Jin K (2014) Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer’s disease. J Alzheimer’s Dis: JAD 38(2):437–444. https://doi.org/10.3233/jad-131124
O’Neill C (2013) PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol 48(7):647–653. https://doi.org/10.1016/j.exger.2013.02.025
Yang F, Chu X, Yin M, Liu X, Yuan H, Niu Y, Fu L (2014) mTOR and autophagy in normal brain aging and caloric restriction ameliorating age-related cognition deficits. Behav Brain Res 264:82–90. https://doi.org/10.1016/j.bbr.2014.02.005
Ryskalin L, Lazzeri G, Flaibani M, Biagioni F, Gambardella S, Frati A, Fornai F (2017) mTOR-dependent cell proliferation in the brain. Biomed Res Int 2017:7082696. https://doi.org/10.1155/2017/7082696
Yates SC, Zafar A, Hubbard P, Nagy S, Durant S, Bicknell R, Wilcock G, Christie S et al (2013) Dysfunction of the mTOR pathway is a risk factor for Alzheimer’s disease. Acta Neuropathol Commun 1:3. https://doi.org/10.1186/2051-5960-1-3
Gouras GK (2012) mTOR: at the crossroads of aging, chaperones, and Alzheimer’s disease. J Neurochem 124(6):747–748. https://doi.org/10.1111/jnc.12098
Caccamo A, Majumder S, Richardson A, Strong R, Oddo S (2010) Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-β, and tau. J Biol Chem 285(17):13107–13120. https://doi.org/10.1074/jbc.m110.100420
Tang Z, Bereczki E, Zhang H, Wang S, Li C, Ji X, Branca RM, Lehtiö J et al (2013) Mammalian target of rapamycin (mTor) mediates tau protein dyshomeostasis. J Biol Chem 288(22):15556–15570. https://doi.org/10.1074/jbc.m112.435123
Oddo S (2013) The relationship among mTOR, beta-amyloid and tau: therapeutic implications for Alzheimer’s disease. Alzheimers Dement 9(4):P315. https://doi.org/10.1016/j.jalz.2013.04.139
Oddo S (2010) Molecular interplay between mTOR, Aβ and tau: effects on cognitive impairments. Alzheimers Dement 6(4):S167. https://doi.org/10.1016/j.jalz.2010.05.519
Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P (2007) mTOR controls mitochondrial oxidative function through a YY1–PGC-1α transcriptional complex. Nature 450(7170):736–740. https://doi.org/10.1038/nature06322
Weichhart T, Haidinger M, Katholnig K, Kopecky C, Poglitsch M, Lassnig C, Rosner M, Zlabinger GJ et al (2011) Inhibition of mTOR blocks the anti-inflammatory effects of glucocorticoids in myeloid immune cells. Blood 117(16):4273–4283. https://doi.org/10.1182/blood-2010-09-310888
Kimball SR, Abbas A, Jefferson LS (2008) Melatonin represses oxidative stress-induced activation of the MAP kinase and mTOR signaling pathways in H4IIE hepatoma cells through inhibition of Ras. J Pineal Res 44(4):379–386. https://doi.org/10.1111/j.1600-079x.2007.00539.x
Jung T, Höhn A, Grune T (2014) The proteasome and the degradation of oxidized proteins: part II—protein oxidation and proteasomal degradation. Redox Biol 2:99–104. https://doi.org/10.1016/j.redox.2013.12.008
Aiken CT, Kaake RM, Wang X, Huang L (2011) Oxidative stress-mediated regulation of proteasome complexes. Mol Cell Proteomics. https://doi.org/10.1074/mcp.r110.006924
Davies KJA (2001) Degradation of oxidized proteins by the 20S proteasome. Biochimie 83(3–4):301–310. https://doi.org/10.1016/s0300-9084(01)01250-0
Bulteau A-L, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI (2001) Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem 276(32):30057–30063. https://doi.org/10.1074/jbc.m100142200
Wang X, Chemmama IE, Yu C, Huszagh A, Xu Y, Viner R, Block SA, Cimermancic P et al (2017) The proteasome-interacting Ecm29 protein disassembles the 26S proteasome in response to oxidative stress. J Biol Chem 292(39):16310–16320. https://doi.org/10.1074/jbc.M117.803619
Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R et al (2008) Dopamine-modified α-synuclein blocks chaperone-mediated autophagy. J Clin Investig. https://doi.org/10.1172/jci32806
Zhang L, Sun Y, Fei M, Tan C, Wu J, Zheng J, Tang J, Sun W et al (2014) Disruption of chaperone-mediated autophagy-dependent degradation of MEF2A by oxidative stress-induced lysosome destabilization. Autophagy 10(6):1015–1035. https://doi.org/10.4161/auto.28477
Wan F-Y, Wang Y-N, Zhang G-J (2001) The influence of oxidation of membrane thiol groups on lysosomal proton permeability. Biochem J 360(2):355. https://doi.org/10.1042/0264-6021:3600355
Pivtoraiko VN, Stone SL, Roth KA, Shacka JJ (2009) Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death. Antioxid Redox Signal 11(3):481–496. https://doi.org/10.1089/ars.2008.2263
Ditaranto K, Tekirian TL, Yang AJ (2001) Lysosomal membrane damage in soluble Aβ-mediated cell death in Alzheimer’s disease. Neurobiol Dis 8(1):19–31. https://doi.org/10.1006/nbdi.2000.0364
Acosta-Cabronero J, Williams GB, Cardenas-Blanco A, Arnold RJ, Lupson V, Nestor PJ (2013) In vivo quantitative susceptibility mapping (QSM) in Alzheimer’s disease. PLoS One 8(11):e81093. https://doi.org/10.1371/journal.pone.0081093
Zhu WZ, Zhong WD, Wang W, Zhan CJ, Wang CY, Qi JP, Wang JZ, Lei T (2009) Quantitative MR phase-corrected imaging to investigate increased brain iron deposition of patients with Alzheimer disease. Radiology 253(2):497–504. https://doi.org/10.1148/radiol.2532082324
Qin Y, Zhu W, Zhan C, Zhao L, Wang J, Tian Q, Wang W (2011) Investigation on positive correlation of increased brain iron deposition with cognitive impairment in Alzheimer disease by using quantitative MR R2’ mapping. J Huazhong Univ Sci Technol Med Sci= Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban 31(4):578–585. https://doi.org/10.1007/s11596-011-0493-1
Smith MA, Zhu X, Tabaton M, Liu G, McKeel DW Jr, Cohen ML, Wang X, Siedlak SL et al (2010) Increased iron and free radical generation in preclinical Alzheimer disease and mild cognitive impairment. J Alzheimer’s Dis: JAD 19(1):363–372. https://doi.org/10.3233/jad-2010-1239
Ganz T, Nemeth E (2012) Hepcidin and iron homeostasis. Biochim Biophys Acta 1823(9):1434–1443. https://doi.org/10.1016/j.bbamcr.2012.01.014
Camaschella C (2013) Iron and hepcidin: a story of recycling and balance. Hematol Am Soc Hematol Educ Program 2013:1–8. https://doi.org/10.1182/asheducation-2013.1.1
Millonig G, Ganzleben I, Peccerella T, Casanovas G, Brodziak-Jarosz L, Breitkopf-Heinlein K, Dick TP, Seitz HK et al (2012) Sustained submicromolar H2O2 levels induce hepcidin via signal transducer and activator of transcription 3 (STAT3). J Biol Chem 287(44):37472–37482. https://doi.org/10.1074/jbc.M112.358911
Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T (2004) IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113(9):1271–1276. https://doi.org/10.1172/jci20945
Pietrangelo A, Dierssen U, Valli L, Garuti C, Rump A, Corradini E, Ernst M, Klein C et al (2007) STAT3 is required for IL-6-gp130-dependent activation of hepcidin in vivo. Gastroenterology 132(1):294–300. https://doi.org/10.1053/j.gastro.2006.10.018
Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU (2007) STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 109(1):353–358. https://doi.org/10.1182/blood-2006-07-033969
Wrighting DM, Andrews NC (2006) Interleukin-6 induces hepcidin expression through STAT3. Blood 108(9):3204–3209. https://doi.org/10.1182/blood-2006-06-027631
Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, Gonzalez-Billault C, Nunez MT (2013) Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem 126(4):541–549. https://doi.org/10.1111/jnc.12244
Wong AD, Ye M, Levy AF, Rothstein JD, Bergles DE, Searson PC (2013) The blood-brain barrier: an engineering perspective. Frontiers Neuroengineering 6:7. https://doi.org/10.3389/fneng.2013.00007
Rathore KI, Redensek A, David S (2012) Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-alpha and TGF-beta1. Glia 60(5):738–750. https://doi.org/10.1002/glia.22303
Qian ZM, He X, Liang T, Wu KC, Yan YC, Lu LN, Yang G, Luo QQ et al (2014) Lipopolysaccharides upregulate hepcidin in neuron via microglia and the IL-6/STAT3 signaling pathway. Mol Neurobiol 50(3):811–820. https://doi.org/10.1007/s12035-014-8671-3
Silva B, Faustino P (2015) An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim Biophys Acta 1852(7):1347–1359. https://doi.org/10.1016/j.bbadis.2015.03.011
Rouault TA (2006) The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat Chem Biol 2(8):406–414. https://doi.org/10.1038/nchembio807
Bornsen L, Romme Christensen J, Ratzer R, Hedegaard C, Sondergaard HB, Krakauer M, Hesse D, Nielsen CH et al (2015) Endogenous interferon-beta-inducible gene expression and interferon-beta-treatment are associated with reduced T cell responses to myelin basic protein in multiple sclerosis. PLoS One 10(3):e0118830. https://doi.org/10.1371/journal.pone.0118830
Eisenstein RS, Ross KL (2003) Novel roles for iron regulatory proteins in the adaptive response to iron deficiency. J Nutr 133(5 Suppl 1):1510s–1516s
Caltagirone A, Weiss G, Pantopoulos K (2001) Modulation of cellular iron metabolism by hydrogen peroxide. Effects of H2O2 on the expression and function of iron-responsive element-containing mRNAs in B6 fibroblasts. J Biol Chem 276(23):19738–19745. https://doi.org/10.1074/jbc.M100245200
Pantopoulos K, Hentze MW (1995) Rapid responses to oxidative stress mediated by iron regulatory protein. EMBO J 14(12):2917–2924
Soum E, Brazzolotto X, Goussias C, Bouton C, Moulis JM, Mattioli TA, Drapier JC (2003) Peroxynitrite and nitric oxide differently target the iron-sulfur cluster and amino acid residues of human iron regulatory protein 1. Biochemistry 42(25):7648–7654. https://doi.org/10.1021/bi030041i
Cairo G, Ronchi R, Recalcati S, Campanella A, Minotti G (2002) Nitric oxide and peroxynitrite activate the iron regulatory protein-1 of J774A.1 macrophages by direct disassembly of the Fe-S cluster of cytoplasmic aconitase. Biochemistry 41(23):7435–7442
Kim S, Ponka P (2002) Nitric oxide-mediated modulation of iron regulatory proteins: Implication for cellular iron homeostasis. Blood Cells Mol Dis 29(3):400–410
Mikhael M, Kim SF, Schranzhofer M, Soe-Lin S, Sheftel AD, Mullner EW, Ponka P (2006) Iron regulatory protein-independent regulation of ferritin synthesis by nitrogen monoxide. FEBS J 273(16):3828–3836. https://doi.org/10.1111/j.1742-4658.2006.05390.x
Pinero DJ, Hu J, Connor JR (2000) Alterations in the interaction between iron regulatory proteins and their iron responsive element in normal and Alzheimer’s diseased brains. Cell Mol Biol (Noisy-le-Grand, France) 46(4):761–776
Altamura S, Muckenthaler MU (2009) Iron toxicity in diseases of aging: Alzheimer’s disease, Parkinson’s disease and atherosclerosis. J Alzheimer’s Dis : JAD 16(4):879–895. https://doi.org/10.3233/jad-2009-1010
Guyant-Marechal L, Rovelet-Lecrux A, Goumidi L, Cousin E, Hannequin D, Raux G, Penet C, Ricard S et al (2007) Variations in the APP gene promoter region and risk of Alzheimer disease. Neurology 68(9):684–687. https://doi.org/10.1212/01.wnl.0000255938.33739.46
Cahill CM, Lahiri DK, Huang X, Rogers JT (2009) Amyloid precursor protein and alpha synuclein translation, implications for iron and inflammation in neurodegenerative diseases. Biochim Biophys Acta 1790(7):615–628. https://doi.org/10.1016/j.bbagen.2008.12.001
Cho HH, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X, Rogers JT (2010) Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J Biol Chem 285(41):31217–31232. https://doi.org/10.1074/jbc.M110.149161
Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, Lahiri DK, Leedman PJ et al (2008) Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer's disease. Biochem Soc Trans 36(Pt 6):1282–1287. https://doi.org/10.1042/bst0361282
Bodovitz S, Falduto MT, Frail DE, Klein WL (1995) Iron levels modulate α-secretase cleavage of amyloid precursor protein. J Neurochem 64(1):307–315. https://doi.org/10.1046/j.1471-4159.1995.64010307.x
Silvestri L, Camaschella C (2008) A potential pathogenetic role of iron in Alzheimer’s disease. J Cell Mol Med 12(5a):1548–1550. https://doi.org/10.1111/j.1582-4934.2008.00356.x
Hwang EM, Kim SK, Sohn JH, Lee JY, Kim Y, Kim YS, Mook-Jung I (2006) Furin is an endogenous regulator of alpha-secretase associated APP processing. Biochem Biophys Res Commun 349(2):654–659. https://doi.org/10.1016/j.bbrc.2006.08.077
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 13. https://doi.org/10.1016/s1474-4422(14)70117-6
Everett J, Cespedes E, Shelford LR, Exley C, Collingwood JF, Dobson J, van der Laan G, Jenkins CA et al (2014) Ferrous iron formation following the co-aggregation of ferric iron and the Alzheimer’s disease peptide -amyloid (1-42). J R Soc Interface 11(95):20140165–20140165. https://doi.org/10.1098/rsif.2014.0165
Everett J, Cespedes E, Shelford LR, Exley C, Collingwood JF, Dobson J, van der Laan G, Jenkins CA et al (2014) Evidence of redox-active iron formation following aggregation of ferrihydrite and the Alzheimer’s disease peptide beta-amyloid. Inorg Chem 53(6):2803–2809. https://doi.org/10.1021/ic402406g
Everett J, Cespedes E, Shelford LR, Exley C, Collingwood JF, Dobson J, van der Laan G, Jenkins CA et al (2014) Ferrous iron formation following the co-aggregation of ferric iron and the Alzheimer’s disease peptide beta-amyloid (1-42). J Royal Soc Interface 11(95):20140165. https://doi.org/10.1098/rsif.2014.0165
Telling ND, Everett J, Collingwood JF, Dobson J, van der Laan G, Gallagher JJ, Wang J, Hitchcock AP (2017) Iron biochemistry is correlated with amyloid plaque morphology in an established mouse model of Alzheimer’s disease. Cell Chem Biol 24(10):1205–1215.e1203. https://doi.org/10.1016/j.chembiol.2017.07.014
Dahms SO, Könnig I, Roeser D, Gührs K-H, Mayer MC, Kaden D, Multhaup G, Than ME (2012) Metal binding dictates conformation and function of the amyloid precursor protein (APP) E2 domain. J Mol Biol 416(3):438–452. https://doi.org/10.1016/j.jmb.2011.12.057
Leskovjan AC, Kretlow A, Lanzirotti A, Miller LM Synchrotron-based imaging detects metal and plaques in a mouse model of Alzheimer’s disease. In: 2007 I.E. 33rd Annual Northeast Bioengineering Conference, 10–11 March 2007 2007. pp 54–55. doi:https://doi.org/10.1109/NEBC.2007.4413276
Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, Chevion M, Perry G et al (2001) Redox-active iron mediates amyloid-β toxicity. Free Radic Biol Med 30(4):447–450. https://doi.org/10.1016/s0891-5849(00)00494-9
Ha C, Ryu J, Park CB (2007) Metal ions differentially influence the aggregation and deposition of Alzheimer’s β-amyloid on a solid template†. Biochemistry 46(20):6118–6125. https://doi.org/10.1021/bi7000032
Cohen SI, Linse S, Luheshi LM, Hellstrand E, White DA, Rajah L, Otzen DE, Vendruscolo M et al (2013) Proliferation of amyloid-beta42 aggregates occurs through a secondary nucleation mechanism. Proc Natl Acad Sci U S A 110(24):9758–9763. https://doi.org/10.1073/pnas.1218402110
Huang X, Atwood CS, Moir RD, Hartshorn MA, Tanzi RE, Bush AI (2004) Trace metal contamination initiates the apparent auto-aggregation, amyloidosis, and oligomerization of Alzheimer’s Abeta peptides. J Biol Inorg Chem: JBIC : a publication of the Society of Biological Inorganic Chemistry 9(8):954–960. https://doi.org/10.1007/s00775-004-0602-8
Liu B, Moloney A, Meehan S, Morris K, Thomas SE, Serpell LC, Hider R, Marciniak SJ et al (2011) Iron promotes the toxicity of amyloid beta peptide by impeding its ordered aggregation. J Biol Chem 286(6):4248–4256. https://doi.org/10.1074/jbc.M110.158980
Hare D, Ayton S, Bush A, Lei P (2013) A delicate balance: iron metabolism and diseases of the brain. Front Aging Neurosci 5(34). https://doi.org/10.3389/fnagi.2013.00034
Guo C, Wang T, Zheng W, Shan Z-Y, Teng W-P, Wang Z-Y (2013) Intranasal deferoxamine reverses iron-induced memory deficits and inhibits amyloidogenic APP processing in a transgenic mouse model of Alzheimer’s disease. Neurobiol Aging 34(2):562–575. https://doi.org/10.1016/j.neurobiolaging.2012.05.009
Lovell MA, Xiong S, Xie C, Davies P, Markesbery WR (2004) Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimer’s Dis: JAD 6(6):659–671 discussion 673-681
Chan A, Shea TB (2006) Dietary and genetically-induced oxidative stress alter tau phosphorylation: influence of folate and apolipoprotein E deficiency. J Alzheimer’s Dis: JAD 9(4):399–405
Munoz P, Zavala G, Castillo K, Aguirre P, Hidalgo C, Nunez MT (2006) Effect of iron on the activation of the MAPK/ERK pathway in PC12 neuroblastoma cells. Biol Res 39(1):189–190
Huang X, Dai J, Huang C, Zhang Q, Bhanot O, Pelle E (2007) Deferoxamine synergistically enhances iron-mediated AP-1 activation: a showcase of the interplay between extracellular-signal-regulated kinase and tyrosine phosphatase. Free Radic Res 41(10):1135–1142. https://doi.org/10.1080/10715760701609061
Guo C, Wang P, Zhong M-L, Wang T, Huang X-S, Li J-Y, Wang Z-Y (2013) Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem Int 62(2):165–172. https://doi.org/10.1016/j.neuint.2012.12.005
Schipper HM, Bennett DA, Liberman A, Bienias JL, Schneider JA, Kelly J, Arvanitakis Z (2006) Glial heme oxygenase-1 expression in Alzheimer disease and mild cognitive impairment. Neurobiol Aging 27(2):252–261. https://doi.org/10.1016/j.neurobiolaging.2005.01.016
Wang D, Hui Y, Peng Y, Tang L, Jin J, He R, Li Y, Zhang S et al (2015) Overexpression of heme oxygenase 1 causes cognitive decline and affects pathways for tauopathy in mice. J Alzheimer’s Dis: JAD 43(2):519–534. https://doi.org/10.3233/jad-140567
Chen J (2014) Heme oxygenase in neuroprotection: from mechanisms to therapeutic implications. Rev Neurosci 25(2):269–280. https://doi.org/10.1515/revneuro-2013-0046
Perry V, Cunningham C, Boche D (2002) Atypical inflammation in the central nervous system in prion disease. Curr Opin Neurol 15:349–354
Ruttkay-Nedecky B, Nejdl L, Gumulec J, Zitka O, Masarik M, Eckschlager T, Stiborova M, Adam V et al (2013) The role of metallothionein in oxidative stress. Int J Mol Sci 14(3):6044–6066. https://doi.org/10.3390/ijms14036044
Suzuki KT, Someya A, Komada Y, Ogra Y (2002) Roles of metallothionein in copper homeostasis: responses to cu-deficient diets in mice. J Inorg Biochem 88(2):173–182. https://doi.org/10.1016/S0162-0134(01)00376-2
Stelmashook EV, Isaev NK, Genrikhs EE, Amelkina GA, Khaspekov LG, Skrebitsky VG, Illarioshkin SN (2014) Role of zinc and copper ions in the pathogenetic mechanisms of Alzheimer’s and Parkinson’s diseases. Biochemistry Biokhimiia 79(5):391–396. https://doi.org/10.1134/s0006297914050022
Nakahata S, Morishita K (2014) PP2A inactivation by ROS accumulation. Blood 124(14):2163–2165. https://doi.org/10.1182/blood-2014-08-594093
Chen L, Liu L, Yin J, Luo Y, Huang S (2009) Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. Int J Biochem Cell Biol 41(6):1284–1295. https://doi.org/10.1016/j.biocel.2008.10.029
Arif M, Kazim SF, Grundke-Iqbal I, Garruto RM, Iqbal K (2014) Tau pathology involves protein phosphatase 2A in parkinsonism-dementia of Guam. Proc Natl Acad Sci 111(3):1144–1149. https://doi.org/10.1073/pnas.1322614111
Zenaro E, Piacentino G, Constantin G (2017) The blood-brain barrier in Alzheimer’s disease. Neurobiol Dis 107:41–56. https://doi.org/10.1016/j.nbd.2016.07.007
Montagne A, Zhao Z, Zlokovic BV (2017) Alzheimer’s disease: a matter of blood-brain barrier dysfunction? J Exp Med 214(11):3151–3169. https://doi.org/10.1084/jem.20171406
Pietronigro E, Zenaro E, Constantin G (2016) Imaging of leukocyte trafficking in Alzheimer’s disease. Front Immunol 7:33. https://doi.org/10.3389/fimmu.2016.00033
Rochfort KD, Cummins PM (2015) The blood-brain barrier endothelium: a target for pro-inflammatory cytokines. Biochem Soc Trans 43(4):702–706. https://doi.org/10.1042/bst20140319
Varatharaj A, Galea I (2017) The blood-brain barrier in systemic inflammation. Brain Behav Immun 60:1–12. https://doi.org/10.1016/j.bbi.2016.03.010
Gray MT, Woulfe JM (2015) Striatal blood–brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab 35(5):747–750. https://doi.org/10.1038/jcbfm.2015.32
Cabezas R, Ávila M, Gonzalez J, El-Bachá RS, Báez E, García-Segura LM, Jurado Coronel JC, Capani F et al (2014) Astrocytic modulation of blood brain barrier: perspectives on Parkinson’s disease. Front Cell Neurosci 8:211. https://doi.org/10.3389/fncel.2014.00211
Qin XY, Zhang SP, Cao C, Loh YP, Cheng Y (2016) Aberrations in peripheral inflammatory cytokine levels in Parkinson disease: a systematic review and meta-analysis. JAMA Neurology 73(11):1316–1324. https://doi.org/10.1001/jamaneurol.2016.2742
Wang Q, Liu Y, Zhou J (2015) Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener 4:19. https://doi.org/10.1186/s40035-015-0042-0
Dick FD, De Palma G, Ahmadi A, Scott NW, Prescott GJ, Bennett J, Semple S, Dick S et al (2007) Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup Environ Med 64(10):666–672. https://doi.org/10.1136/oem.2006.027003
Lill CM, Klein C (2017) Epidemiology and causes of Parkinson’s disease. Nervenarzt 88(4):345–355. https://doi.org/10.1007/s00115-017-0288-0
Acknowledgements
We should like to thank Victoria Storey for her kind assistance with the figures.
Authorships
All authors contributed to the writing up of the paper.
Funding
MB is supported by a NHMRC Senior Principal Research Fellowship (APP1059660).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Morris, G., Berk, M., Maes, M. et al. Could Alzheimer’s Disease Originate in the Periphery and If So How So?. Mol Neurobiol 56, 406–434 (2019). https://doi.org/10.1007/s12035-018-1092-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1092-y