Abstract
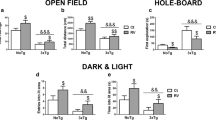
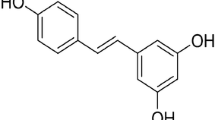
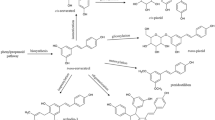
Resveratrol (RSV) is a natural compound present in berries, grapes and red wine that has shown some neuroprotective properties, but the mechanism by which RSV exhibits its protective role is not very well understood yet. Little is known about the effect of RSV on adenosinergic system, a system regulated in an age-dependent manner in SAMP8 mice, widely considered as an Alzheimer’s model. Therefore, the aim of the present work was to assess whether RSV intake was able to modulate the adenosine-mediated signalling in SAMP8 mice. Data showed herein clearly demonstrate the ability of RSV to modulate adenosine receptor gene expression as well as transduction pathway mediated by receptors expressed on plasma membrane. Interestingly, this polyphenol was able to reverse the age-related loss of adenosine A1 receptors and its corresponding signalling pathway. Moreover, adenosine A2A receptors were not modulated by aging or RSV, but A2A-mediated signalling was completely desensitized after RSV treatment compared to untreated mice. Enzymes involved on adenosine metabolism, such as 5′-nucleotidase and adenosine deaminase, were found to be reduced after RSV treatment, but adenosine levels remained unchanged. Nevertheless, an age-related decrease on 5′-nucleotidase activity and adenosine and related metabolite levels was observed. In conclusion, our data show that RSV modulates adenosine-mediated signalling, strongly suggesting that the role of RSV via adenosine receptor signalling and its modulation of neurotransmission in neurodegenerative diseases should be considered as new therapeutic target for RSV neuroprotective effect.








Similar content being viewed by others
References
Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J (2001) International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev 53(4):527–552
Alonso-Andres P, Albasanz JL, Ferrer I, Martin M (2018) Purine-related metabolites and their converting enzymes are altered in frontal, parietal and temporal cortex at early stages of Alzheimer's disease pathology. Brain Pathol. https://doi.org/10.1111/bpa.12592
Borea PA, Gessi S, Merighi S, Varani K (2016) Adenosine as a multi-signalling Guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol Sci 37(6):419–434. https://doi.org/10.1016/j.tips.2016.02.006
Antonioli L, Blandizzi C, Csoka B, Pacher P, Hasko G (2015) Adenosine signalling in diabetes mellitus—pathophysiology and therapeutic considerations. Nat Rev Endocrinol 11(4):228–241. https://doi.org/10.1038/nrendo.2015.10
Ribeiro JA, Sebastiao AM, de Mendonca A (2002) Adenosine receptors in the nervous system: pathophysiological implications. Prog Neurobiol 68(6):377–392
Cunha RA (2016) How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem 139:1019–1055. https://doi.org/10.1111/jnc.13724
Albasanz JL, Perez S, Barrachina M, Ferrer I, Martin M (2008) Up-regulation of adenosine receptors in the frontal cortex in Alzheimer's disease. Brain Pathol 18(2):211–219. https://doi.org/10.1111/j.1750-3639.2007.00112.x
Villar-Menendez I, Porta S, Buira SP, Pereira-Veiga T, Diaz-Sanchez S, Albasanz JL, Ferrer I, Martin M et al (2014) Increased striatal adenosine A2A receptor levels is an early event in Parkinson's disease-related pathology and it is potentially regulated by miR-34b. Neurobiol Dis 69:206–214. https://doi.org/10.1016/j.nbd.2014.05.030
Villar-Menendez I, Blanch M, Tyebji S, Pereira-Veiga T, Albasanz JL, Martin M, Ferrer I, Perez-Navarro E et al (2013) Increased 5-methylcytosine and decreased 5-hydroxymethylcytosine levels are associated with reduced striatal A2AR levels in Huntington's disease. NeuroMolecular Med 15(2):295–309. https://doi.org/10.1007/s12017-013-8219-0
Albasanz JL, Rodriguez A, Ferrer I, Martin M (2006) Adenosine A2A receptors are up-regulated in Pick's disease frontal cortex. Brain Pathol 16(4):249–255. https://doi.org/10.1111/j.1750-3639.2006.00026.x
Albasanz JL, Rodriguez A, Ferrer I, Martin M (2007) Up-regulation of adenosine A1 receptors in frontal cortex from Pick's disease cases. Eur J Neurosci 26(12):3501–3508. https://doi.org/10.1111/j.1460-9568.2007.05965.x
Castillo CA, Albasanz JL, Leon D, Jordan J, Pallas M, Camins A, Martin M (2009) Age-related expression of adenosine receptors in brain from the senescence-accelerated mouse. Exp Gerontol 44(6–7):453–461. https://doi.org/10.1016/j.exger.2009.04.006
Wei X, Zhang Y, Zhou J (1999) Alzheimer's disease-related gene expression in the brain of senescence accelerated mouse. Neurosci Lett 268(3):139–142
Angulo E, Casado V, Mallol J, Canela EI, Vinals F, Ferrer I, Lluis C, Franco R (2003) A1 adenosine receptors accumulate in neurodegenerative structures in Alzheimer disease and mediate both amyloid precursor protein processing and tau phosphorylation and translocation. Brain Pathol 13(4):440–451
Fukumitsu N, Ishii K, Kimura Y, Oda K, Hashimoto M, Suzuki M, Ishiwata K (2008) Adenosine A(1) receptors using 8-dicyclopropylmethyl-1-[(11)C]methyl-3-propylxanthine PET in Alzheimer's disease. Ann Nucl Med 22(10):841–847. https://doi.org/10.1007/s12149-008-0185-5
Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, Guo W, Kang J et al (2015) Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci 18(3):423–434. https://doi.org/10.1038/nn.3930
Baur JA, Sinclair DA (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov 5(6):493–506. https://doi.org/10.1038/nrd2060
Syarifah-Noratiqah S, Naina-Mohamed I, Zulfarina MS, Qodriyah HM (2017) Natural polyphenols in the treatment of Alzheimer's disease. Curr Drug Targets 19:927–937. https://doi.org/10.2174/1389450118666170328122527
Bhandari R, Kuhad A (2017) Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem Int 103:8–23. https://doi.org/10.1016/j.neuint.2016.12.012
Cianciulli A, Dragone T, Calvello R, Porro C, Trotta T, Lofrumento DD, Panaro MA (2015) IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int Immunopharmacol 24(2):369–376. https://doi.org/10.1016/j.intimp.2014.12.035
Moussa C, Hebron M, Huang X, Ahn J, Rissman RA, Aisen PS, Turner RS (2017) Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer's disease. J Neuroinflammation 14(1):1. https://doi.org/10.1186/s12974-016-0779-0
Ge L, Liu L, Liu H, Liu S, Xue H, Wang X, Yuan L, Wang Z et al (2015) Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol 768:49–57. https://doi.org/10.1016/j.ejphar.2015.10.026
Rangarajan P, Karthikeyan A, Dheen ST (2016) Role of dietary phenols in mitigating microglia-mediated neuroinflammation. NeuroMolecular Med 18(3):453–464. https://doi.org/10.1007/s12017-016-8430-x
Palomera-Avalos V, Grinan-Ferre C, Izquierdo V, Camins A, Sanfeliu C, Pallas M (2017) Metabolic stress induces cognitive disturbances and inflammation in aged mice: protective role of resveratrol. Rejuvenation Res 20(3):202–217. https://doi.org/10.1089/rej.2016.1885
Marambaud P, Zhao H, Davies P (2005) Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem 280(45):37377–37382. https://doi.org/10.1074/jbc.M508246200
Jia Y, Wang N, Liu X (2017) Resveratrol and amyloid-beta: mechanistic insights. Nutrients 9(10). https://doi.org/10.3390/nu9101122
Gehm BD, McAndrews JM, Chien PY, Jameson JL (1997) Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc Natl Acad Sci U S A 94(25):14138–14143
El-Mowafy AM, Alkhalaf M (2003) Resveratrol activates adenylyl-cyclase in human breast cancer cells: a novel, estrogen receptor-independent cytostatic mechanism. Carcinogenesis 24(5):869–873
Park SJ, Ahmad F, Philp A, Baar K, Williams T, Luo H, Ke H, Rehmann H et al (2012) Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148(3):421–433. https://doi.org/10.1016/j.cell.2012.01.017
Tillu DV, Melemedjian OK, Asiedu MN, Qu N, De Felice M, Dussor G, Price TJ (2012) Resveratrol engages AMPK to attenuate ERK and mTOR signaling in sensory neurons and inhibits incision-induced acute and chronic pain. Mol Pain 8:5. https://doi.org/10.1186/1744-8069-8-5
Razali N, Agarwal R, Agarwal P, Kumar S, Tripathy M, Vasudevan S, Crowston JG, Ismail NM (2015) Role of adenosine receptors in resveratrol-induced intraocular pressure lowering in rats with steroid-induced ocular hypertension. Clin Exp Ophthalmol 43(1):54–66. https://doi.org/10.1111/ceo.12375
Gupta YK, Chaudhary G, Srivastava AK (2002) Protective effect of resveratrol against pentylenetetrazole-induced seizures and its modulation by an adenosinergic system. Pharmacology 65(3):170–174
Leon D, Albasanz JL, Ruiz MA, Fernandez M, Martin M (2002) Adenosine A1 receptor down-regulation in mothers and fetal brain after caffeine and theophylline treatments to pregnant rats. J Neurochem 82(3):625–634
Giust D, Da Ros T, Martin M, Albasanz JL (2014) [60]fullerene derivative modulates adenosine and metabotropic glutamate receptors gene expression: a possible protective effect against hypoxia. J Nanobiotechnol 12:27. https://doi.org/10.1186/s12951-014-0027-7
Leon DA, Castillo CA, Albasanz JL, Martin M (2009) Reduced expression and desensitization of adenosine A1 receptor/adenylyl cyclase pathway after chronic (−)N6-phenylisopropyladenosine intake during pregnancy. Neuroscience 163(2):524–532. https://doi.org/10.1016/j.neuroscience.2009.06.050
Leon-Navarro DA, Albasanz JL, Martin M (2015) Hyperthermia-induced seizures alter adenosine A1 and A2A receptors and 5′-nucleotidase activity in rat cerebral cortex. J Neurochem 134(3):395–404. https://doi.org/10.1111/jnc.13130
Dal-Pan A, Pifferi F, Marchal J, Picq JL, Aujard F, Consortium R (2011) Cognitive performances are selectively enhanced during chronic caloric restriction or resveratrol supplementation in a primate. PLoS One 6(1):e16581. https://doi.org/10.1371/journal.pone.0016581
Wang R, Zhang Y, Li J, Zhang C (2017) Resveratrol ameliorates spatial learning memory impairment induced by Abeta1-42 in rats. Neuroscience 344:39–47. https://doi.org/10.1016/j.neuroscience.2016.08.051
Akiguchi I, Pallas M, Budka H, Akiyama H, Ueno M, Han J, Yagi H, Nishikawa T et al (2017) SAMP8 mice as a neuropathological model of accelerated brain aging and dementia: Toshio Takeda's legacy and future directions. Neuropathology 37(4):293–305. https://doi.org/10.1111/neup.12373
Cheng J, Rui Y, Qin L, Xu J, Han S, Yuan L, Yin X, Wan Z (2017) Vitamin D combined with resveratrol prevents cognitive decline in SAMP8 mice. Curr Alzheimer Res 14(8):820–833. https://doi.org/10.2174/1567205014666170207093455
Porquet D, Casadesus G, Bayod S, Vicente A, Canudas AM, Vilaplana J, Pelegri C, Sanfeliu C et al (2013) Dietary resveratrol prevents Alzheimer's markers and increases life span in SAMP8. Age (Dordr) 35(5):1851–1865. https://doi.org/10.1007/s11357-012-9489-4
Grinan-Ferre C, Palomera-Avalos V, Puigoriol-Illamola D, Camins A, Porquet D, Pla V, Aguado F, Pallas M (2016) Behaviour and cognitive changes correlated with hippocampal neuroinflammaging and neuronal markers in female SAMP8, a model of accelerated senescence. Exp Gerontol 80:57–69. https://doi.org/10.1016/j.exger.2016.03.014
Mazzanti G, Di Giacomo S (2016) Curcumin and resveratrol in the management of cognitive disorders: what is the clinical evidence? Molecules 21(9). doi:https://doi.org/10.3390/molecules21091243
Turner RS, Thomas RG, Craft S, van Dyck CH, Mintzer J, Reynolds BA, Brewer JB, Rissman RA et al (2015) A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 85(16):1383–1391. https://doi.org/10.1212/WNL.0000000000002035
Palomera-Avalos V, Grinan-Ferre C, Puigoriol-Ilamola D, Camins A, Sanfeliu C, Canudas AM, Pallas M (2017) Resveratrol protects SAMP8 brain under metabolic stress: focus on mitochondrial function and Wnt pathway. Mol Neurobiol 54(3):1661–1676. https://doi.org/10.1007/s12035-016-9770-0
Folbergrova J, Jesina P, Kubova H, Otahal J (2018) Effect of resveratrol on oxidative stress and mitochondrial dysfunction in immature brain during epileptogenesis. Mol Neurobiol. https://doi.org/10.1007/s12035-018-0924-0
Jardim FR, de Rossi FT, Nascimento MX, da Silva Barros RG, Borges PA, Prescilio IC, de Oliveira MR (2017) Resveratrol and brain mitochondria: a review. Mol Neurobiol 55:2085–2101. https://doi.org/10.1007/s12035-017-0448-z
Rui Y, Cheng J, Qin L, Shan C, Chang J, Wang G, Wan Z (2017) Effects of vitamin D and resveratrol on metabolic associated markers in liver and adipose tissue from SAMP8 mice. Exp Gerontol 93:16–28. https://doi.org/10.1016/j.exger.2017.03.017
Andre DM, Calixto MC, Sollon C, Alexandre EC, Leiria LO, Tobar N, Anhe GF, Antunes E (2016) Therapy with resveratrol attenuates obesity-associated allergic airway inflammation in mice. Int Immunopharmacol 38:298–305. https://doi.org/10.1016/j.intimp.2016.06.017
Fischer-Posovszky P, Kukulus V, Tews D, Unterkircher T, Debatin KM, Fulda S, Wabitsch M (2010) Resveratrol regulates human adipocyte number and function in a Sirt1-dependent manner. Am J Clin Nutr 92(1):5–15. https://doi.org/10.3945/ajcn.2009.28435
Burnstock G, Gentile D (2018) The involvement of purinergic signalling in obesity. Purinergic Signal 14(2):97–108. https://doi.org/10.1007/s11302-018-9605-8
Milton-Laskibar I, Gomez-Zorita S, Aguirre L, Fernandez-Quintela A, Gonzalez M, Portillo MP (2017) Resveratrol-induced effects on body fat differ depending on feeding conditions. Molecules 22(12). https://doi.org/10.3390/molecules22122091
Fernandez-Quintela A, Carpene C, Fernandez M, Aguirre L, Milton-Laskibar I, Contreras J, Portillo MP (2016) Anti-obesity effects of resveratrol: comparison between animal models and humans. J Physiol Biochem 73(3):417–429. https://doi.org/10.1007/s13105-016-0544-y
Lange KW, Li S (2018) Resveratrol, pterostilbene, and dementia. Biofactors 44(1):83–90. https://doi.org/10.1002/biof.1396
Chiang MC, Nicol CJ, Cheng YC (2017) Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem Int 115:1–10. https://doi.org/10.1016/j.neuint.2017.10.002
Molino S, Dossena M, Buonocore D, Ferrari F, Venturini L, Ricevuti G, Verri M (2016) Polyphenols in dementia: from molecular basis to clinical trials. Life Sci 161:69–77. https://doi.org/10.1016/j.lfs.2016.07.021
Liu Y, Beyer A, Aebersold R (2016) On the dependency of cellular protein levels on mRNA abundance. Cell 165(3):535–550. https://doi.org/10.1016/j.cell.2016.03.014
Polycarpou E, Meira LB, Carrington S, Tyrrell E, Modjtahedi H, Carew MA (2013) Resveratrol 3-O-D-glucuronide and resveratrol 4'-O-D-glucuronide inhibit colon cancer cell growth: evidence for a role of A3 adenosine receptors, cyclin D1 depletion, and G1 cell cycle arrest. Mol Nutr Food Res 57(10):1708–1717. https://doi.org/10.1002/mnfr.201200742
Mishina M, Kimura Y, Sakata M, Ishii K, Oda K, Toyohara J, Kimura K, Ishiwata K (2017) Age-related decrease in male extra-striatal adenosine A1 receptors measured using(11)C-MPDX PET. Front Pharmacol 8:903. https://doi.org/10.3389/fphar.2017.00903
Ekonomou A, Pagonopoulou O, Angelatou F (2000) Age-dependent changes in adenosine A1 receptor and uptake site binding in the mouse brain: an autoradiographic study. J Neurosci Res 60(2):257–265. https://doi.org/10.1002/(SICI)1097-4547(20000415)60:2<257::AID-JNR15>3.0.CO;2-U
Meerlo P, Roman V, Farkas E, Keijser JN, Nyakas C, Luiten PG (2004) Ageing-related decline in adenosine A1 receptor binding in the rat brain: an autoradiographic study. J Neurosci Res 78(5):742–748. https://doi.org/10.1002/jnr.20314
Stockwell J, Jakova E, Cayabyab FS (2017) Adenosine A1 and A2A receptors in the brain: current research and their role in neurodegeneration. Molecules 22(4). doi:https://doi.org/10.3390/molecules22040676
Snyder DL, Wang W, Pelleg A, Friedman E, Horwitz J, Roberts J (1998) Effect of aging on A1-adenosine receptor-mediated inhibition of norepinephrine release in the rat heart. J Cardiovasc Pharmacol 31(3):352–358
Ashton KJ, Nilsson U, Willems L, Holmgren K, Headrick JP (2003) Effects of aging and ischemia on adenosine receptor transcription in mouse myocardium. Biochem Biophys Res Commun 312(2):367–372
Garcia-Esparcia P, Hernandez-Ortega K, Ansoleaga B, Carmona M, Ferrer I (2015) Purine metabolism gene deregulation in Parkinson's disease. Neuropathol Appl Neurobiol 41(7):926–940. https://doi.org/10.1111/nan.12221
Viana da Silva S, Haberl MG, Zhang P, Bethge P, Lemos C, Goncalves N, Gorlewicz A, Malezieux M et al (2016) Early synaptic deficits in the APP/PS1 mouse model of Alzheimer's disease involve neuronal adenosine A2A receptors. Nat Commun 7:11915. https://doi.org/10.1038/ncomms11915
Li P, Rial D, Canas PM, Yoo JH, Li W, Zhou X, Wang Y, van Westen GJ et al (2015) Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol Psychiatry 20(11):1339–1349. https://doi.org/10.1038/mp.2014.182
Kolahdouzan M, Hamadeh MJ (2017) The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci Ther 23(4):272–290. https://doi.org/10.1111/cns.12684
Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tane J et al (2009) Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer's disease mice. J Alzheimers Dis 17(3):661–680. https://doi.org/10.3233/JAD-2009-1087
Cunha RA (2005) Neuroprotection by adenosine in the brain: from a(1) receptor activation to a (2A) receptor blockade. Purinergic Signal 1(2):111–134. https://doi.org/10.1007/s11302-005-0649-1
Canas PM, Porciuncula LO, Cunha GM, Silva CG, Machado NJ, Oliveira JM, Oliveira CR, Cunha RA (2009) Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta-amyloid peptides via p38 mitogen-activated protein kinase pathway. J Neurosci 29(47):14741–14751. https://doi.org/10.1523/JNEUROSCI.3728-09.2009
Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J (2006) Caffeine protects Alzheimer's mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience 142(4):941–952. https://doi.org/10.1016/j.neuroscience.2006.07.021
Madeira MH, Elvas F, Boia R, Goncalves FQ, Cunha RA, Ambrosio AF, Santiago AR (2015) Adenosine A2AR blockade prevents neuroinflammation-induced death of retinal ganglion cells caused by elevated pressure. J Neuroinflammation 12:115. https://doi.org/10.1186/s12974-015-0333-5
Rebola N, Simoes AP, Canas PM, Tome AR, Andrade GM, Barry CE, Agostinho PM, Lynch MA et al (2011) Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J Neurochem 117(1):100–111. https://doi.org/10.1111/j.1471-4159.2011.07178.x
Santiago AR, Baptista FI, Santos PF, Cristovao G, Ambrosio AF, Cunha RA, Gomes CA (2014) Role of microglia adenosine a(2A) receptors in retinal and brain neurodegenerative diseases. Mediat Inflamm 2014:465694–465613. https://doi.org/10.1155/2014/465694
Voloshyna I, Hai O, Littlefield MJ, Carsons S, Reiss AB (2013) Resveratrol mediates anti-atherogenic effects on cholesterol flux in human macrophages and endothelium via PPARgamma and adenosine. Eur J Pharmacol 698(1–3):299–309. https://doi.org/10.1016/j.ejphar.2012.08.024
Guixa-Gonzalez R, Albasanz JL, Rodriguez-Espigares I, Pastor M, Sanz F, Marti-Solano M, Manna M, Martinez-Seara H et al (2017) Membrane cholesterol access into a G-protein-coupled receptor. Nat Commun 8:14505. https://doi.org/10.1038/ncomms14505
Albasanz JL, Dalfo E, Ferrer I, Martin M (2005) Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer's disease and dementia with Lewy bodies correlates with stage of Alzheimer's-disease-related changes. Neurobiol Dis 20(3):685–693. https://doi.org/10.1016/j.nbd.2005.05.001
Dalfo E, Albasanz JL, Rodriguez A, Martin M, Ferrer I (2005) Abnormal group I metabotropic glutamate receptor expression and signaling in the frontal cortex in pick disease. J Neuropathol Exp Neurol 64(7):638–647
Rodriguez-Perdigon M, Tordera RM, Gil-Bea FJ, Gerenu G, Ramirez MJ, Solas M (2016) Down-regulation of glutamatergic terminals (VGLUT1) driven by Abeta in Alzheimer's disease. Hippocampus 26(10):1303–1312. https://doi.org/10.1002/hipo.22607
Holmes C, Smith H, Ganderton R, Arranz M, Collier D, Powell J, Lovestone S (2001) Psychosis and aggression in Alzheimer's disease: the effect of dopamine receptor gene variation. J Neurol Neurosurg Psychiatry 71(6):777–779
Seeman P (2010) Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses 4(1):56–73. https://doi.org/10.3371/CSRP.4.1.5
Gardoni F, Di Luca M (2006) New targets for pharmacological intervention in the glutamatergic synapse. Eur J Pharmacol 545(1):2–10. https://doi.org/10.1016/j.ejphar.2006.06.022
de Almeida LM, Pineiro CC, Leite MC, Brolese G, Tramontina F, Feoli AM, Gottfried C, Goncalves CA (2007) Resveratrol increases glutamate uptake, glutathione content, and S100B secretion in cortical astrocyte cultures. Cell Mol Neurobiol 27(5):661–668. https://doi.org/10.1007/s10571-007-9152-2
Li Z, You Z, Li M, Pang L, Cheng J, Wang L (2017) Protective effect of resveratrol on the brain in a rat model of epilepsy. Neurosci Bull 33(3):273–280. https://doi.org/10.1007/s12264-017-0097-2
Ethemoglu MS, Seker FB, Akkaya H, Kilic E, Aslan I, Erdogan CS, Yilmaz B (2017) Anticonvulsant activity of resveratrol-loaded liposomes in vivo. Neuroscience 357:12–19. https://doi.org/10.1016/j.neuroscience.2017.05.026
Pallas M, Ortuno-Sahagun D, Benito-Andres P, Ponce-Regalado MD, Rojas-Mayorquin AE (2014) Resveratrol in epilepsy: preventive or treatment opportunities? Front Biosci (Landmark Ed) 19:1057–1064
Ruiz MA, Leon DA, Albasanz JL, Martin M (2011) Desensitization of adenosine a(1) receptors in rat immature cortical neurons. Eur J Pharmacol 670(2–3):365–371. https://doi.org/10.1016/j.ejphar.2011.09.027
Ruiz MA, Albasanz JL, Leon D, Ros M, Andres A, Martin M (2005) Different modulation of inhibitory and stimulatory pathways mediated by adenosine after chronic in vivo agonist exposure. Brain Res 1031(2):211–221. https://doi.org/10.1016/j.brainres.2004.10.040
Chen JF (2014) Adenosine receptor control of cognition in normal and disease. Int Rev Neurobiol 119:257–307. https://doi.org/10.1016/B978-0-12-801022-8.00012-X
Schmatz R, Schetinger MR, Spanevello RM, Mazzanti CM, Stefanello N, Maldonado PA, Gutierres J, Correa Mde C et al (2009) Effects of resveratrol on nucleotide degrading enzymes in streptozotocin-induced diabetic rats. Life Sci 84(11–12):345–350. https://doi.org/10.1016/j.lfs.2008.12.019
Acknowledgements
This work has been supported by grants SAF2016-33307 from Ministerio de Economía y Competitividad to Mercè Pallas and PEII-2014-030-P from Junta de Comunidades de Castilla-La Mancha (JCCM) to Mairena Martín. Alejandro Sánchez-Melgar is the recipient of a postdoctoral fellowship (PRE-8002/2014) from JCCM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Supplemental Figure 1
Analysis of correlations within adenosinergic system in 5 months old mice. Components previously analyzed belonging to the adenosinergic system were correlated by using Pearson correlation as described in Methods. r. Pearson’s correlation coefficient. P. P value. Straight line: linear regression fit of Pearson’s correlation coefficient value. (PDF 60 kb)
Supplemental Figure 2
Analysis of correlations within adenosinergic system in 7 months old mice. Components previously analyzed belonging to the adenosinergic system were correlated by using Pearson correlation as described in Methods. r. Pearson’s correlation coefficient. P. P value. Straight line: linear regression fit of Pearson’s correlation coefficient value. (PDF 63 kb)
Rights and permissions
About this article
Cite this article
Sánchez-Melgar, A., Albasanz, J.L., Palomera-Ávalos, V. et al. Resveratrol Modulates and Reverses the Age-Related Effect on Adenosine-Mediated Signalling in SAMP8 Mice. Mol Neurobiol 56, 2881–2895 (2019). https://doi.org/10.1007/s12035-018-1281-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-1281-8