Abstract
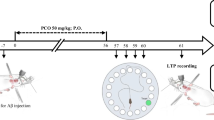
Alzheimer’s disease (AD), the most common form of dementia, is characterized by a progressive decline in cognitive performance and memory formation. The present study was designed to investigate the effect of policosanol (PCO) on cognitive function, oxidative-antioxidative status, and amyloid-beta (Aβ) plaque formation in an AD rat model induced by intracerebroventricular (ICV) injection of Aβ1–40. Healthy adult male Wistar rats were randomly divided into seven groups: control, sham (5 μL, ICV injection of phosphate-buffered saline), AD model (5 μL, ICV injection of Aβ), acacia gum (50 mg/kg, 8 weeks, gavage), PCO (50 mg/kg, 8 weeks, gavage), AD + acacia gum (50 mg/kg, 8 weeks, gavage), and AD + PCO (50 mg/kg, 8 weeks, gavage). During the ninth and tenth weeks of the study, the cognitive function of the rats was assessed by commonly used behavioral paradigms. Subsequently, oxidative-antioxidative status was examined in the serum. Moreover, compact Aβ plaques were detected by Congo red staining. The results showed that injection of Aβ impaired recognition memory in the novel object recognition test, reduced the spatial cognitive ability in the Morris water maze, and alleviated retention and recall capability in the passive avoidance task. Additionally, injection of Aβ resulted in increased total oxidant status, decreased total antioxidant capacity, and enhanced Aβ plaque formation in the rats. Intriguingly, PCO treatment improved all the above-mentioned neuropathological changes in the Aβ-induced AD rats. The results suggest that PCO improves Aβ-induced cognitive decline, possibly through modulation of oxidative-antioxidative status and inhibition of Aβ plaque formation.







Similar content being viewed by others
Data Availability
All relevant data and material are within the manuscript and its supporting information files.
References
Ramachandran AK, Das S, Joseph A, Shenoy GG, Alex AT, Mudgal J (2021) Neurodegenerative pathways in Alzheimer’s disease: a review. Curr Neuropharmacol 19:679–692
Wenk GL (2003) Neuropathologic changes in Alzheimer’s disease. J Clin Psychiatry 64:7–10
Huang H-C, Jiang Z-F (2009) Accumulated amyloid-β peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer’s disease. J Alzheimer’s dis 16:15–27
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 1:a006189
Yan Y, Yang H, Xie Y, Ding Y, Kong D, Yu H (2020) Research progress on Alzheimer’s disease and resveratrol. Neurochem Res 45:989–1006
Belviranlı M, Okudan N (2019) Voluntary, involuntary and forced exercises almost equally reverse behavioral impairment by regulating hippocampal neurotrophic factors and oxidative stress in experimental Alzheimer’s disease model. Behav Brain Res 364:245–255
Abeysinghe A, Deshapriya R, Udawatte C (2020) Alzheimer’s disease; a review of the pathophysiological basis and therapeutic interventions. Life Sci 256:117996
Ionescu-Tucker A, Cotman CW (2021) Emerging roles of oxidative stress in brain aging and Alzheimer’s disease. Neurobiol Aging 107:86–95
Bai R, Guo J, Ye XY, Xie Y, Xie T (2022) Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev 77:101619
Halliwell B (2006) Oxidative stress and neurodegeneration: where are we now? J Neurochem 97:1634–1658
Lee CC, Wu DY, Chen SY, Lin YP, Lee TM (2021) Exercise intensities modulate cognitive function in spontaneously hypertensive rats through oxidative mediated synaptic plasticity in hippocampus. J Cell Mol Med 25:8546–8557
Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24:1583
Rummel NG, Butterfield DA (2022) Altered metabolism in Alzheimer disease brain: role of oxidative stress. Antioxid Redox Signal 36:1289–1305
Uddin M, Kabir M (2019) Oxidative stress in Alzheimer’s disease: molecular hallmarks of underlying vulnerability. In: Biological, Diagnostic and Therapeutic Advances in Alzheimer's Disease: Springer, p 91–115.
Aliev G, Obrenovich ME, Reddy VP, Shenk JC, Moreira PI, Nunomura A, Zhu X, Smith MA, Perry G (2008) Antioxidant therapy in Alzheimer’s disease: theory and practice. Mini Rev Med Chem 8:1395–1406
Tadokoro K, Ohta Y, Inufusa H, Loon AFN, Abe K (2020) Prevention of cognitive decline in Alzheimer’s disease by novel antioxidative supplements. Int J Mol Sci 21:1974
Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y (2020) Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener 15:40
Chen JX, Yan SD (2007) Amyloid-beta-induced mitochondrial dysfunction. J Alzheimers Dis 12:177–184
Misrani A, Tabassum S, Yang L (2021) Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease. Front Aging Neurosci 13:617588
Ferreira ST, Klein WL (2011) The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol Learn Mem 96:529–543
Rashno M, Gholipour P, Salehi I, Komaki A, Rashidi K, Khoshnam SE, Ghaderi S (2022) p-Coumaric acid mitigates passive avoidance memory and hippocampal synaptic plasticity impairments in aluminum chloride-induced Alzheimer’s disease rat model. J Funct Foods 94:105117
Shen J, Luo F, Lin Q (2019) Policosanol: extraction and biological functions. J Funct Foods 57:351–360
Musto D, Martorelli L, Russo M, Esposito G, Amato M, Esposito P, Riegler G (2010) Non-alcoholic hepatic steatosis: the role of policosanols in associated hyperlipidemia. Minerva Gastroenterol Dietol 56:389–395
Gong J, Qin X, Yuan F, Hu M, Chen G, Fang K, Wang D, Jiang S, Li J, Zhao Y (2018) Efficacy and safety of sugarcane policosanol on dyslipidemia: a meta-analysis of randomized controlled trials. Mol Nutr Food Res 62:1700280
Dulin MF, Hatcher LF, Sasser HC, Barringer TA (2006) Policosanol is ineffective in the treatment of hypercholesterolemia: a randomized controlled trial. Am J Clin Nutr 84:1543–1548
Sun L, Li X, Ma C, He Z, Zhang X, Wang C, Zhao M, Gan J, Feng Y (2022) Improving effect of the policosanol from Ericerus pela wax on learning and memory impairment caused by scopolamine in mice. Foods 11:2095
Zhang X, Ma C, Sun L, He Z, Feng Y, Li X, Gan J, Chen X (2021) Effect of policosanol from insect wax on amyloid β-peptide-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer’s disease. BMC Complement Med Ther 21:103
Ma J, Li K, Zhang W, Ma L, Xu J, Liu L, Chen X, Zhang H (2022) Acute toxicity and chromosomal aberration toxicity of insect wax and its policosanol. Food Sci Human Wellness 11:356–365
Guerra YP, Cuevas VM, Ferreiro RM, Yera AO, Despaigne SJ (2015) Effects of policosanol pre-treatment on blood-brain barrier damage induced by ischemia-reperfusion in rats. Int J Pharm Sci Rev Res 32:1–6
Elseweidy MM, Zein N, Aldhamy SE, Elsawy MM, Saeid SA (2016) Policosanol as a new inhibitor candidate for vascular calcification in diabetic hyperlipidemic rats. Exp Biol Med 241:1943–1949
Asadbegi M, Komaki A, Salehi I, Yaghmaei P, Ebrahim-Habibi A, Shahidi S, Sarihi A, Soleimani Asl S, Golipoor Z (2018) Effects of thymol on amyloid-β-induced impairments in hippocampal synaptic plasticity in rats fed a high-fat diet. Brain Res Bull 137:338–350
Lorenzo A, Yankner BA (1994) Beta-amyloid neurotoxicity requires fibril formation and is inhibited by Congo red. Proc Natl Acad Sci U S A 91:12243–12247
Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates: Elsevier Academic Press. San Diego, CA
Zarrinkalam E, Heidarianpour A, Salehi I, Ranjbar K, Komaki A (2016) Effects of endurance, resistance, and concurrent exercise on learning and memory after morphine withdrawal in rats. Life Sci 157:19–24
Benzie IF, Strain J (1999) [2] Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol 299:15–27
Erel O (2005) A new automated colorimetric method for measuring total oxidant status. Clin Biochem 38:1103–1111
Ahmadi N, Safari S, Mirazi N, Karimi SA, Komaki A (2021) Effects of vanillic acid on Aβ(1–40)-induced oxidative stress and learning and memory deficit in male rats. Brain Res Bull 170:264–273
Park J, Lee SY, Shon J, Kim K, Lee HJ, Kim KA, Lee BY, Oh SH, Kim NK, Kim OJ (2019) Adalimumab improves cognitive impairment, exerts neuroprotective effects and attenuates neuroinflammation in an Aβ(1–40)-injected mouse model of Alzheimer’s disease. Cytotherapy 21:671–682
Ahmadi N, Mirazi N, Komaki A, Safari S, Hosseini A (2021) Vanillic acid attenuates amyloid β1-40-induced long-term potentiation deficit in male rats: an in vivo investigation. Neurol Res 43:562–569
Prediger RD, Franco JL, Pandolfo P, Medeiros R, Duarte FS, Di Giunta G, Figueiredo CP, Farina M, Calixto JB, Takahashi RN, Dafre AL (2007) Differential susceptibility following beta-amyloid peptide-(1–40) administration in C57BL/6 and Swiss albino mice: evidence for a dissociation between cognitive deficits and the glutathione system response. Behav Brain Res 177:205–213
Sun L, Li X, Ma C, He Z, Zhang X, Wang C, Zhao M, Gan J, Feng Y (2022) Improving effect of the policosanol from ericerus pela wax on learning and memory impairment caused by scopolamine in mice. Foods 11:2095
Fontani G, Lodi L, Migliorini S, Corradeschi F (2009) Effect of omega-3 and policosanol supplementation on attention and reactivity in athletes. J Am Coll Nutr 28(Suppl):473s–481s
Betteridge DJ (2000) What is oxidative stress? Metab 49:3–8
Garbarino VR, Orr ME, Rodriguez KA, Buffenstein R (2015) Mechanisms of oxidative stress resistance in the brain: lessons learned from hypoxia tolerant extremophilic vertebrates. Arch Biochem Biophys 576:8–16
Gholipour P, Komaki A, Parsa H, Ramezani M (2022) Therapeutic effects of high-intensity interval training exercise alone and its combination with ecdysterone against amyloid beta-induced rat model of Alzheimer’s disease: a behavioral, biochemical, and histological study. Neurochem Res 47:2090–2108
Gholipour P, Komaki A, Ramezani M, Parsa H (2022) Effects of the combination of high-intensity interval training and Ecdysterone on learning and memory abilities, antioxidant enzyme activities, and neuronal population in an amyloid-beta-induced rat model of Alzheimer’s disease. Physiol Behav 251:113817
Hasanzadeh Z, Nourazarian A, Nikanfar M, Laghousi D, Vatankhah AM, Sadrirad S (2021) Evaluation of the serum Dkk-1, tenascin-C, oxidative stress markers levels and Wnt signaling pathway genes expression in patients with Alzheimer’s disease. J Mol Neurosci 71:879–887
Franzoni F, Scarfò G, Guidotti S, Fusi J, Asomov M, Pruneti C (2021) Oxidative stress and cognitive decline: the neuroprotective role of natural antioxidants. Front Neurosci 15:729757
Erel O (2004) A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem 37:277–285
Cakirca G, Manav V, Celik H, Saracoglu G, Yetkin EN (2020) Effects of anxiety and depression symptoms on oxidative stress in patients with alopecia areata. Postepy Dermatol Alergol 37:412–416
Wang T, Liu YY, Wang X, Yang N, Zhu HB, Zuo PP (2010) Protective effects of octacosanol on 6-hydroxydopamine-induced Parkinsonism in rats via regulation of ProNGF and NGF signaling. Acta Pharmacol Sin 31:765–774
Molina V, Ravelo Y, Noa M, Mas R, Pérez Y, Oyarzábal A, Mendoza N, Valle M, Jiménez S, Sánchez J (2013) Therapeutic effects of policosanol and atorvastatin against global brain ischaemia-reperfusion injury in gerbils. Indian J Pharm Sci 75:635–641
Rahman MM, Lendel C (2021) Extracellular protein components of amyloid plaques and their roles in Alzheimer’s disease pathology. Mol Neurodegener 16:59
Araki W, Kametani F (2022) Protection against amyloid-β oligomer neurotoxicity by small molecules with antioxidative properties: potential for the prevention of Alzheimer’s disease dementia. Antioxidants 11:132
Simunkova M, Alwasel SH, Alhazza IM, Jomova K, Kollar V, Rusko M, Valko M (2019) Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch Toxicol 93:2491–2513
Calvo-Rodriguez M, Hou SS, Snyder AC, Kharitonova EK, Russ AN, Das S, Fan Z, Muzikansky A, Garcia-Alloza M, Serrano-Pozo A, Hudry E, Bacskai BJ (2020) Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat Commun 11:2146
Cascella R, Cecchi C (2021) Calcium Dyshomeostasis in Alzheimer’s Disease Pathogenesis. Int J Mol Sci 22:4914
Oliver DMA, Reddy PH (2019) Small molecules as therapeutic drugs for Alzheimer’s disease. Mol Cell Neurosci 96:47–62
Giordano CR, Terlecky LJ, Bollig-Fischer A, Walton PA, Terlecky SR (2014) Amyloid-beta neuroprotection mediated by a targeted antioxidant. Sci Rep 4:4983
Manczak M, Mao P, Calkins MJ, Cornea A, Reddy AP, Murphy MP, Szeto HH, Park B, Reddy PH (2010) Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J Alzheimers Dis 20(Suppl 2):S609-631
Ono K, Hamaguchi T, Naiki H, Yamada M (2006) Anti-amyloidogenic effects of antioxidants: implications for the prevention and therapeutics of Alzheimer’s disease. Biochim Biophys Acta 1762:575–586
Rajasekhar K, Samanta S, Bagoband V, Murugan NA, Govindaraju T (2020) Antioxidant berberine-derivative inhibits multifaceted amyloid toxicity. iScience 23:101005
Tramutola A, Lanzillotta C, Perluigi M, Butterfield DA (2017) Oxidative stress, protein modification and Alzheimer disease. Brain Res Bull 133:88–96
Marsillach J, Adorni MP, Zimetti F, Papotti B, Zuliani G, Cervellati C (2020) HDL proteome and Alzheimer’s disease: evidence of a link. Antioxidants 9:1224
Wingo TS, Cutler DJ, Wingo AP, Le NA, Rabinovici GD, Miller BL, Lah JJ, Levey AI (2019) Association of early-onset Alzheimer disease with elevated low-density lipoprotein cholesterol levels and rare genetic coding variants of APOB. JAMA Neurol 76:809–817
Lewis TL, Cao D, Lu H, Mans RA, Su YR, Jungbauer L, Linton MF, Fazio S, LaDu MJ, Li L (2010) Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. J Biol Chem 285:36958–36968
Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA (2010) Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol 67:1491–1497
Zhou Z, Liang Y, Zhang X, Xu J, Lin J, Zhang R, Kang K, Liu C, Zhao C, Zhao M (2020) Low-density lipoprotein cholesterol and Alzheimer’s disease: a systematic review and meta-analysis. Front Aging Neurosci 12:5
Castaño G, Menéndez R, Más R, Amor A, Fernández JL, González RL, Lezcay M, Alvarez E (2002) Effects of policosanol and lovastatin on lipid profile and lipid peroxidation in patients with dyslipidemia associated with type 2 diabetes mellitus. Int J Clin Pharmacol Res 22:89–99
Gong J, Qin X, Yuan F, Hu M, Chen G, Fang K, Wang D, Jiang S, Li J, Zhao Y (2018) Efficacy and safety of sugarcane policosanol on dyslipidemia: A meta‐analysis of randomized controlled trials. Mol Nutri Food Res 62:1700280
Acknowledgements
The authors are grateful to the staff of the Neurophysiology Research Center, Hamadan University of Medical Sciences, for the role they had in doing this project.
Funding
The current study was funded (Grant No. IR.BASU.REC.1399.371) by the Faculty of Basic Sciences, Bu-Ali Sina University, Hamedan, Iran.
Author information
Authors and Affiliations
Contributions
Samaneh Safari: study concept and design, data acquisition, data analysis, interpretation, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and statistical analysis.
Naser Mirazi: supervision, conceptualization, writing, review and editing, and data curation.
Nesa Ahmadi: preparation of the original draft and resources.
Masoumeh Asadbegi: data analysis and interpretation, formal analysis, and software validation.
Alireza Nourian: method validation.
Shahab Ghaderi: data analysis and revision of the manuscript.
Masome Rashno: data analysis and revision of the manuscript.
Alireza Komaki: study concept and design, critical revision of the manuscript for important intellectual content, and study supervision.
Corresponding author
Ethics declarations
Ethics Approval
According to the Guidelines of the National Institutes of Health, the experiments were carried out on the principles of laboratory animal care (NIH Publication 80–23, 1996). The Local Ethical Committee approved all planned experimental procedures.
Consent to Participate
Because this research has been done on animal models, “Consent to Participate” is not relevant.
Consent for Publication
All authors read and approved the final manuscript. All authors of this article are completely satisfied with its publication.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This paper was carried out as a part of Samaneh Safari’s MSc thesis.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Safari, S., Mirazi, N., Ahmadi, N. et al. The Protective Effects of Policosanol on Learning and Memory Impairments in a Male Rat Model of Alzheimer’s Disease. Mol Neurobiol 60, 2507–2519 (2023). https://doi.org/10.1007/s12035-023-03225-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03225-x