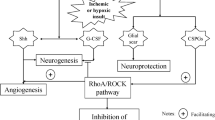
Abstract
Ischemic stroke is one of the main reasons of disability and death. Stroke-induced functional deficits are mainly due to the secondary degeneration of the white matter characterized by axonal demyelination and injury of axon-glial integrity. Enhancement of the axonal regeneration and remyelination could promote the neural functional recovery. However, cerebral ischemia-induced activation of RhoA/Rho kinase (ROCK) pathway plays a crucial and harmful role in the process of axonal recovery and regeneration. Inhibition of this pathway could promote the axonal regeneration and remyelination. In addition, hydrogen sulfide (H2S) has the significant neuroprotective role during the recovery of ischemic stroke via inhibiting the inflammatory response and oxidative stress, regulating astrocyte function, promoting the differentiation of endogenous oligodendrocyte precursor cells (OPCs) to mature oligodendrocyte. Among all of these effects, promoting the formation of mature oligodendrocyte is a crucial part of axonal regeneration and remyelination. Furthermore, numerous studies have uncovered the crosstalk between astrocytes and oligodendrocyte, microglial cells and oligodendrocyte in the axonal remyelination following ischemic stroke. The purpose of this review was to discuss the relationship among H2S, RhoA/ROCK pathway, astrocytes, and microglial cells in the axonal remyelination following ischemic stroke to reveal new strategies for preventing and treating this devastating disease.




Similar content being viewed by others
Data Availability
Not applicable.
References
Powers WJ (2020) Acute ischemic stroke. N Engl J Med 383(3):252–260. https://doi.org/10.1056/NEJMcp1917030
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR et al (2020) Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 141(9):e139–e596. https://doi.org/10.1161/CIR.0000000000000757
Gallacher KI, Jani BD, Hanlon P, Nicholl BI, Mair FS (2019) Multimorbidity in stroke. Stroke 50(7):1919–1926. https://doi.org/10.1161/STROKEAHA.118.020376
Caprio FZ, Sorond FA (2019) Cerebrovascular disease: primary and secondary stroke prevention. Med Clin North Am 103(2):295–308. https://doi.org/10.1016/j.mcna.2018.10.001
Moskowitz MA, Lo EH, Iadecola C (2010) The science of stroke: mechanisms in search of treatments. Neuron 67(2):181–198. https://doi.org/10.1016/j.neuron.2010.07.002
Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4(5):399–415. https://doi.org/10.1038/nrn1106
Garcia-Martin G, Alcover-Sanchez B, Wandosell F, Cubelos B (2022) Pathways involved in remyelination after cerebral ischemia. Curr Neuropharmacol 20(4):751–765. https://doi.org/10.2174/1570159X19666210610093658
Mattos DJS, Rutlin J, Hong X, Zinn K, Shimony JS, Carter AR (2021) White matter integrity of contralesional and transcallosal tracts may predict response to upper limb task-specific training in chronic stroke. Neuroimage Clin 31:102710. https://doi.org/10.1016/j.nicl.2021.102710
Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A, Basser P (2001) Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. Neuroimage 13(6 Pt 1):1174–1185. https://doi.org/10.1006/nimg.2001.0765
Jindahra P, Petrie A, Plant GT (2012) The time course of retrograde trans-synaptic degeneration following occipital lobe damage in humans. Brain 135(Pt 2):534–541. https://doi.org/10.1093/brain/awr324
Marin MA, Carmichael ST (2019) Mechanisms of demyelination and remyelination in the young and aged brain following white matter stroke. Neurobiol Dis 126:5–12. https://doi.org/10.1016/j.nbd.2018.07.023
Thomalla G, Glauche V, Koch MA, Beaulieu C, Weiller C, Rother J (2004) Diffusion tensor imaging detects early Wallerian degeneration of the pyramidal tract after ischemic stroke. Neuroimage 22(4):1767–1774. https://doi.org/10.1016/j.neuroimage.2004.03.041
Tobin MK, Stephen TKL, Lopez KL, Pergande MR, Bartholomew AM, Cologna SM, Lazarov O (2020) Activated mesenchymal stem cells induce recovery following stroke via regulation of inflammation and oligodendrogenesis. J Am Heart Assoc 9(7):e013583. https://doi.org/10.1161/JAHA.119.013583
Blasi F, Wei Y, Balkaya M, Tikka S, Mandeville JB, Waeber C, Ayata C, Moskowitz MA (2014) Recognition memory impairments after subcortical white matter stroke in mice. Stroke 45(5):1468–1473. https://doi.org/10.1161/STROKEAHA.114.005324
Symons M, Settleman J (2000) Rho family GTPases: more than simple switches. Trends Cell Biol 10(10):415–419. https://doi.org/10.1016/s0962-8924(00)01832-8
Wen JY, Gao SS, Chen FL, Chen S, Wang M, Chen ZW (2019) Role of CSE-Produced H2S on cerebrovascular relaxation via RhoA-ROCK inhibition and cerebral ischemia-reperfusion injury in mice. ACS Chem Neurosci 10(3):1565–1574. https://doi.org/10.1021/acschemneuro.8b00533
Chen H, Firestein BL (2007) RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J Neurosci 27(31):8378–8386. https://doi.org/10.1523/JNEUROSCI.0872-07.2007
Zhang Y, Li K, Wang X, Ding Y, Ren Z, Fang J, Sun T, Guo Y et al (2021) CSE-derived H2S inhibits reactive astrocytes proliferation and promotes neural functional recovery after cerebral ischemia/reperfusion injury in mice via inhibition of RhoA/ROCK2 pathway. ACS Chem Neurosci 12(14):2580–2590. https://doi.org/10.1021/acschemneuro.0c00674
Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E, Fasudil Ischemic Stroke Study G (2005) Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci 238(1–2):31–39. https://doi.org/10.1016/j.jns.2005.06.003
Li X, Zhang KY, Zhang P, Chen LX, Wang L, Xie M, Wang CY, Tang XQ (2014) Hydrogen sulfide inhibits formaldehyde-induced endoplasmic reticulum stress in PC12 cells by upregulation of SIRT-1. PLoS One 9(2):e89856. https://doi.org/10.1371/journal.pone.0089856
Qu K, Lee SW, Bian JS, Low CM, Wong PT (2008) Hydrogen sulfide: neurochemistry and neurobiology. Neurochem Int 52(1–2):155–165. https://doi.org/10.1016/j.neuint.2007.05.016
Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16(13):1792–1798. https://doi.org/10.1096/fj.02-0211hyp
Von Bartheld CS, Bahney J, Herculano-Houzel S (2016) The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comparative Neurol 524(18):3865–3895. https://doi.org/10.1002/cne.24040
Pelvig DP, Pakkenberg H, Stark AK, Pakkenberg B (2008) Neocortical glial cell numbers in human brains. Neurobiol Aging 29(11):1754–1762. https://doi.org/10.1016/j.neurobiolaging.2007.04.013
Pekny M, Wilhelmsson U, Tatlisumak T, Pekna M (2019) Astrocyte activation and reactive gliosis-A new target in stroke? Neurosci Lett 689:45–55. https://doi.org/10.1016/j.neulet.2018.07.021
Mifsud G, Zammit C, Muscat R, Di Giovanni G, Valentino M (2014) Oligodendrocyte pathophysiology and treatment strategies in cerebral ischemia. CNS Neurosci Ther 20(7):603–612. https://doi.org/10.1111/cns.12263
Amantea D, Micieli G, Tassorelli C, Cuartero MI, Ballesteros I, Certo M, Moro MA, Lizasoain I et al (2015) Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front Neurosci 9:147. https://doi.org/10.3389/fnins.2015.00147
Shibahara T,Ago T,Nakamura K,Tachibana M,Yoshikawa Y,Komori M,Yamanaka K,Wakisaka Y et al (2020) Pericyte-mediated tissue repair through PDGFRbeta promotes peri-infarct astrogliosis, oligodendrogenesis, and functional recovery after acute ischemic stroke. eNeuro 7(2). https://doi.org/10.1523/ENEURO.0474-19.2020
Raffaele S, Fumagalli M (2022) Dynamics of microglia activation in the ischemic brain: implications for myelin repair and functional recovery. Front Cell Neurosci 16:950819. https://doi.org/10.3389/fncel.2022.950819
Regenold WT, Phatak P, Marano CM, Gearhart L, Viens CH, Hisley KC (2007) Myelin staining of deep white matter in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and unipolar major depression. Psychiatry Res 151(3):179–188. https://doi.org/10.1016/j.psychres.2006.12.019
Chang KJ, Redmond SA, Chan JR (2016) Remodeling myelination: implications for mechanisms of neural plasticity. Nat Neurosci 19(2):190–197. https://doi.org/10.1038/nn.4200
Saab AS, Tzvetanova ID, Nave KA (2013) The role of myelin and oligodendrocytes in axonal energy metabolism. Curr Opin Neurobiol 23(6):1065–1072. https://doi.org/10.1016/j.conb.2013.09.008
Mecha M, Yanguas-Casas N, Feliu A, Mestre L, Carrillo-Salinas F, Azcoitia I, Yong VW, Guaza C (2019) The endocannabinoid 2-AG enhances spontaneous remyelination by targeting microglia. Brain Behav Immun 77:110–126. https://doi.org/10.1016/j.bbi.2018.12.013
Jian Z, Liu R, Zhu X, Smerin D, Zhong Y, Gu L, Fang W, Xiong X (2019) The involvement and therapy target of immune cells after ischemic stroke. Front Immunol 10:2167. https://doi.org/10.3389/fimmu.2019.02167
Eldahshan W, Fagan SC, Ergul A (2019) Inflammation within the neurovascular unit: focus on microglia for stroke injury and recovery. Pharmacol Res 147:104349. https://doi.org/10.1016/j.phrs.2019.104349
Koch JC, Tonges L, Barski E, Michel U, Bahr M, Lingor P (2014) ROCK2 is a major regulator of axonal degeneration, neuronal death and axonal regeneration in the CNS. Cell Death Dis 5:e1225. https://doi.org/10.1038/cddis.2014.191
Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF (2017) Pathogenic mechanisms following ischemic stroke. Neurol Sci 38(7):1167–1186. https://doi.org/10.1007/s10072-017-2938-1
Orellana-Urzua S, Rojas I, Libano L, Rodrigo R (2020) Pathophysiology of ischemic stroke: role of oxidative stress. Curr Pharm Des 26(34):4246–4260. https://doi.org/10.2174/1381612826666200708133912
Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar-Milovanovic E, Zafirovic S, Mousad SA, Isenovic ER (2017) Apoptosis and acute brain ischemia in ischemic stroke. Curr Vasc Pharmacol 15(2):115–122. https://doi.org/10.2174/1570161115666161104095522
Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD (2019) Global brain inflammation in stroke. Lancet Neurol 18(11):1058–1066. https://doi.org/10.1016/S1474-4422(19)30078-X
Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA (2019) Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation 16(1):142. https://doi.org/10.1186/s12974-019-1516-2
Gomes-Leal W (2019) Why microglia kill neurons after neural disorders? The friendly fire hypothesis. Neural Regen Res 14(9):1499–1502. https://doi.org/10.4103/1673-5374.255359
Miyanohara J, Kakae M, Nagayasu K, Nakagawa T, Mori Y, Arai K, Shirakawa H, Kaneko S (2018) TRPM2 channel aggravates CNS inflammation and cognitive impairment via activation of microglia in chronic cerebral hypoperfusion. J Neurosci 38(14):3520–3533. https://doi.org/10.1523/JNEUROSCI.2451-17.2018
Kalakh S, Mouihate A (2017) Demyelination-induced inflammation attracts newly born neurons to the white matter. Mol Neurobiol 54(8):5905–5918. https://doi.org/10.1007/s12035-016-0127-5
Cunha MI,Su M,Cantuti-Castelvetri L,Muller SA,Schifferer M,Djannatian M,Alexopoulos I,Van Der Meer F et al (2020) Pro-inflammatory activation following demyelination is required for myelin clearance and oligodendrogenesis. Journal of Experimental Medicine 217(5). https://doi.org/10.1084/jem.20191390
Tonges L,Koch JC,Bahr M,Lingor P (2011) ROCKing regeneration: Rho kinase inhibition as molecular target for neurorestoration. Frontiers in Molecular Neuroscience 4(39. https://doi.org/10.3389/fnmol.2011.00039
Wang QM, Liao JK (2012) ROCKs as immunomodulators of stroke. Expert Opin Ther Targets 16(10):1013–1025. https://doi.org/10.1517/14728222.2012.715149
Bruzzone B,De Leo P,Sticchi L,Canepa P,Rappazzo E,Anselmo M,Icardi G (2017) Diagnostic and therapeutic implications in a case of mixed hepatitis C virus (HCV) infection. Hepat Mon 17(3). https://doi.org/10.5812/hepatmon.44774
Zhang Y, Miao L, Peng Q, Fan X, Song W, Yang B, Zhang P, Liu G et al (2022) Parthenolide modulates cerebral ischemia-induced microglial polarization and alleviates neuroinflammatory injury via the RhoA/ROCK pathway. Phytomedicine 105:154373. https://doi.org/10.1016/j.phymed.2022.154373
Sladojevic N, Yu B, Liao JK (2017) ROCK as a therapeutic target for ischemic stroke. Expert Rev Neurother 17(12):1167–1177. https://doi.org/10.1080/14737175.2017.1395700
Fujita Y, Yamashita T (2014) Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci 8:338. https://doi.org/10.3389/fnins.2014.00338
Jeon BT, Jeong EA, Park SY, Son H, Shin HJ, Lee DH, Kim HJ, Kang SS et al (2013) The Rho-kinase (ROCK) inhibitor Y-27632 protects against excitotoxicity-induced neuronal death in vivo and in vitro. Neurotox Res 23(3):238–248. https://doi.org/10.1007/s12640-012-9339-2
Cen LP, Liang JJ, Chen JH, Harvey AR, Ng TK, Zhang M, Pang CP, Cui Q et al (2017) AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience 343:472–482. https://doi.org/10.1016/j.neuroscience.2016.12.027
Liu C, Han S, Zheng J, Wang H, Li S, Li J (2022) EphA4 regulates white matter remyelination after ischemic stroke through Ephexin-1/RhoA/ROCK signaling pathway. Glia 70(10):1971–1991. https://doi.org/10.1002/glia.24232
Zheng J, Zhang T, Han S, Liu C, Liu M, Li S, Li J (2021) Activin A improves the neurological outcome after ischemic stroke in mice by promoting oligodendroglial ACVR1B-mediated white matter remyelination. Exp Neurol 337:113574. https://doi.org/10.1016/j.expneurol.2020.113574
Moyon S, Ma D, Huynh JL, Coutts DJC, Zhao C, Casaccia P, Franklin RJM (2017) Efficient Remyelination Requires DNA Methylation. eNeuro 4(2). https://doi.org/10.1523/ENEURO.0336-16.2017
Fernandez-Enright F, Andrews JL, Newell KA, Pantelis C, Huang XF (2014) Novel implications of Lingo-1 and its signaling partners in schizophrenia. Transl Psychiatry 4:e348. https://doi.org/10.1038/tp.2013.121
Bove RM, Green AJ (2017) Remyelinating pharmacotherapies in multiple sclerosis. Neurotherapeutics 14(4):894–904. https://doi.org/10.1007/s13311-017-0577-0
Mi S, Sandrock A, Miller RH (2008) LINGO-1 and its role in CNS repair. Int J Biochem Cell Biol 40(10):1971–1978. https://doi.org/10.1016/j.biocel.2008.03.018
Zhu J, Zhu Z, Ren Y, Dong Y, Li Y, Yang X (2021) LINGO-1 shRNA protects the brain against ischemia/reperfusion injury by inhibiting the activation of NF-kappaB and JAK2/STAT3. Hum Cell 34(4):1114–1122. https://doi.org/10.1007/s13577-021-00527-x
Foale S, Berry M, Logan A, Fulton D, Ahmed Z (2017) LINGO-1 and AMIGO3, potential therapeutic targets for neurological and dysmyelinating disorders? Neural Regen Res 12(8):1247–1251. https://doi.org/10.4103/1673-5374.213538
Meabon JS, De Laat R, Ieguchi K, Serbzhinsky D, Hudson MP, Huber BR, Wiley JC, Bothwell M (2016) Intracellular LINGO-1 negatively regulates Trk neurotrophin receptor signaling. Mol Cell Neurosci 70:1–10. https://doi.org/10.1016/j.mcn.2015.11.002
Li B, Xu Y, Quan Y, Cai Q, Le Y, Ma T, Liu Z, Wu G et al (2020) Inhibition of RhoA/ROCK pathway in the early stage of hypoxia ameliorates depression in mice via protecting myelin sheath. ACS Chem Neurosci 11(17):2705–2716. https://doi.org/10.1021/acschemneuro.0c00352
Staugaitis SM, Chang A, Trapp BD (2012) Cortical pathology in multiple sclerosis: experimental approaches to studies on the mechanisms of demyelination and remyelination. Acta Neurologica Scandinavica. Supplementum 195:97–102. https://doi.org/10.1111/ane.12041
Chang A, Staugaitis SM, Dutta R, Batt CE, Easley KE, Chomyk AM, Yong VW, Fox RJ et al (2012) Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol 72(6):918–926. https://doi.org/10.1002/ana.23693
Kirby L, Jin J, Cardona JG, Smith MD, Martin KA, Wang J, Strasburger H, Herbst L et al (2019) Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 10(1):3887. https://doi.org/10.1038/s41467-019-11638-3
Song J, Goetz BD, Baas PW, Duncan ID (2001) Cytoskeletal reorganization during the formation of oligodendrocyte processes and branches. Mol Cell Neurosci 17(4):624–636. https://doi.org/10.1006/mcne.2001.0974
Wang H, Rusielewicz T, Tewari A, Leitman EM, Einheber S, Melendez-Vasquez CV (2012) Myosin II is a negative regulator of oligodendrocyte morphological differentiation. J Neurosci Res 90(8):1547–1556. https://doi.org/10.1002/jnr.23036
Xu S, Lu J, Shao A, Zhang JH, Zhang J (2020) Glial cells: role of the immune response in ischemic stroke. Front Immunol 11:294. https://doi.org/10.3389/fimmu.2020.00294
Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA (2010) Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia 58(13):1610–1619. https://doi.org/10.1002/glia.21033
Pantoni L, Garcia JH, Gutierrez JA (1996) Cerebral white matter is highly vulnerable to ischemia. Stroke 27(9):1641–1646; discussion 1647. https://doi.org/10.1161/01.str.27.9.1641
Dyck SM, Alizadeh A, Santhosh KT, Proulx EH, Wu CL, Karimi-Abdolrezaee S (2015) Chondroitin sulfate proteoglycans negatively modulate spinal cord neural precursor cells by signaling through LAR and RPTPsigma and modulation of the Rho/ROCK pathway. Stem Cells 33(8):2550–2563. https://doi.org/10.1002/stem.1979
Dyck S, Kataria H, Akbari-Kelachayeh K, Silver J, Karimi-Abdolrezaee S (2019) LAR and PTPsigma receptors are negative regulators of oligodendrogenesis and oligodendrocyte integrity in spinal cord injury. Glia 67(1):125–145. https://doi.org/10.1002/glia.23533
Pedraza CE, Taylor C, Pereira A, Seng M, Tham CS, Izrael M, Webb M (2014) Induction of oligodendrocyte differentiation and in vitro myelination by inhibition of rho-associated kinase. ASN Neuro 6(4). https://doi.org/10.1177/1759091414538134
Ding Y, Liu B, Zhang Y, Fang F, Li X, Wang S, Wen J (2022) Hydrogen sulphide protects mice against the mutual aggravation of cerebral ischaemia/reperfusion injury and colitis. Eur J Pharmacol 914:174682. https://doi.org/10.1016/j.ejphar.2021.174682
Kimura Y, Shibuya N, Kimura H (2019) Sulfite protects neurons from oxidative stress. Br J Pharmacol 176(4):571–582. https://doi.org/10.1111/bph.14373
Jiang WW, Huang BS, Han Y, Deng LH, Wu LX (2017) Sodium hydrosulfide attenuates cerebral ischemia/reperfusion injury by suppressing overactivated autophagy in rats. FEBS Open Bio 7(11):1686–1695. https://doi.org/10.1002/2211-5463.12301
Zhou X, Cao Y, Ao G, Hu L, Liu H, Wu J, Wang X, Jin M et al (2014) CaMKKbeta-dependent activation of AMP-activated protein kinase is critical to suppressive effects of hydrogen sulfide on neuroinflammation. Antioxid Redox Signal 21(12):1741–1758. https://doi.org/10.1089/ars.2013.5587
Hu LF, Wong PT, Moore PK, Bian JS (2007) Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem 100(4):1121–1128. https://doi.org/10.1111/j.1471-4159.2006.04283.x
Chen Y, Wen J, Chen Z (2021) H2S protects hippocampal neurons against hypoxia-reoxygenation injury by promoting RhoA phosphorylation at Ser188. Cell Death Discov 7(1):132. https://doi.org/10.1038/s41420-021-00514-z
Tarui T, Fukami K, Nagasawa K, Yoshida S, Sekiguchi F, Kawabata A (2010) Involvement of Src kinase in T-type calcium channel-dependent neuronal differentiation of NG108-15 cells by hydrogen sulfide. J Neurochem 114(2):512–519. https://doi.org/10.1111/j.1471-4159.2010.06774.x
Wang Z, Liu DX, Wang FW, Zhang Q, Du ZX, Zhan JM, Yuan QH, Ling EA et al (2013) L-Cysteine promotes the proliferation and differentiation of neural stem cells via the CBS/H(2)S pathway. Neuroscience 237:106–117. https://doi.org/10.1016/j.neuroscience.2012.12.057
Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J (2016) Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke. Transl Stroke Res 7(3):209–219. https://doi.org/10.1007/s12975-016-0459-5
Wei SW, Zou MM, Huan J, Li D, Zhang PF, Lu MH, Xiong J, Ma YX (2022) Role of the hydrogen sulfide-releasing donor ADT-OH in the regulation of mammal neural precursor cells. J Cell Physiol 237(7):2877–2887. https://doi.org/10.1002/jcp.30726
Anrather J, Iadecola C (2016) Inflammation and stroke: an overview. Neurotherapeutics 13(4):661–670. https://doi.org/10.1007/s13311-016-0483-x
Ben Haim L, Rowitch DH (2017) Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci 18(1):31–41. https://doi.org/10.1038/nrn.2016.159
Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S et al (2017) Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med 23(7):818–828. https://doi.org/10.1038/nm.4354
Mukherjee N, Nandi S, Garg S, Ghosh S, Ghosh S, Samat R, Ghosh S (2020) Targeting chondroitin sulfate proteoglycans: an emerging therapeutic strategy to treat CNS injury. ACS Chem Neurosci 11(3):231–232. https://doi.org/10.1021/acschemneuro.0c00004
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 11(1):56–64. https://doi.org/10.1038/nrneurol.2014.207
Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE et al (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541(7638):481–487. https://doi.org/10.1038/nature21029
Jiang T, Luo J, Pan X, Zheng H, Yang H, Zhang L, Hu X (2021) Physical exercise modulates the astrocytes polarization, promotes myelin debris clearance and remyelination in chronic cerebral hypoperfusion rats. Life Sci 278:119526. https://doi.org/10.1016/j.lfs.2021.119526
Jia J, Yang L, Chen Y, Zheng L, Chen Y, Xu Y, Zhang M (2021) The role of microglial phagocytosis in ischemic stroke. Frontiers in Immunology 12:790201. https://doi.org/10.3389/fimmu.2021.790201
De Waard DM, Bugiani M (2020) Astrocyte-oligodendrocyte-microglia crosstalk in astrocytopathies. Front Cell Neurosci 14:608073. https://doi.org/10.3389/fncel.2020.608073
Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A, Hwang P, Chan AT et al (2020) Negative feedback control of neuronal activity by microglia. Nature 586(7829):417–423. https://doi.org/10.1038/s41586-020-2777-8
Li T, Liu T, Chen X, Li L, Feng M, Zhang Y, Wan L, Zhang C et al (2020) Microglia induce the transformation of A1/A2 reactive astrocytes via the CXCR7/PI3K/Akt pathway in chronic post-surgical pain. J Neuroinflammation 17(1):211. https://doi.org/10.1186/s12974-020-01891-5
Shindo A, Liang AC, Maki T, Miyamoto N, Tomimoto H, Lo EH, Arai K (2016) Subcortical ischemic vascular disease: roles of oligodendrocyte function in experimental models of subcortical white-matter injury. J Cereb Blood Flow Metab 36(1):187–198. https://doi.org/10.1038/jcbfm.2015.80
Miyamoto N, Magami S, Inaba T, Ueno Y, Hira K, Kijima C, Nakajima S, Yamashiro K et al (2020) The effects of A1/A2 astrocytes on oligodendrocyte linage cells against white matter injury under prolonged cerebral hypoperfusion. Glia 68(9):1910–1924. https://doi.org/10.1002/glia.23814
Mathys H, Davila-Velderrain J, Peng Z, Gao F, Mohammadi S, Young JZ, Menon M, He L et al (2019) Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570(7761):332–337. https://doi.org/10.1038/s41586-019-1195-2
Escartin C, Galea E, Lakatos A, O’callaghan JP, Petzold GC, Serrano-Pozo A, Steinhauser C, Volterra A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24(3):312–325. https://doi.org/10.1038/s41593-020-00783-4
Lambertsen KL, Finsen B, Clausen BH (2019) Post-stroke inflammation-target or tool for therapy? Acta Neuropathol 137(5):693–714. https://doi.org/10.1007/s00401-018-1930-z
Clausen BH, Lambertsen KL, Babcock AA, Holm TH, Dagnaes-Hansen F, Finsen B (2008) Interleukin-1beta and tumor necrosis factor-alpha are expressed by different subsets of microglia and macrophages after ischemic stroke in mice. J Neuroinflammation 5:46. https://doi.org/10.1186/1742-2094-5-46
Tsuyama J, Nakamura A, Ooboshi H, Yoshimura A, Shichita T (2018) Pivotal role of innate myeloid cells in cerebral post-ischemic sterile inflammation. Seminars in Immunopathology 40(6):523–538. https://doi.org/10.1007/s00281-018-0707-8
Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19(8):987–991. https://doi.org/10.1038/nn.4338
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J (2012) Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43(11):3063–3070. https://doi.org/10.1161/STROKEAHA.112.659656
Ma Y, Wang J, Wang Y, Yang GY (2017) The biphasic function of microglia in ischemic stroke. Prog Neurobiol 157:247–272. https://doi.org/10.1016/j.pneurobio.2016.01.005
Bain JM, Moore L, Ren Z, Simonishvili S, Levison SW (2013) Vascular endothelial growth factors A and C are induced in the SVZ following neonatal hypoxia-ischemia and exert different effects on neonatal glial progenitors. Transl Stroke Res 4(2):158–170. https://doi.org/10.1007/s12975-012-0213-6
Gelosa P, Bonfanti E, Castiglioni L, Delgado-Garcia JM, Gruart A, Fontana L, Gotti M, Tremoli E et al (2019) Improvement of fiber connectivity and functional recovery after stroke by montelukast, an available and safe anti-asthmatic drug. Pharmacol Res 142:223–236. https://doi.org/10.1016/j.phrs.2019.02.025
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, Van Wijngaarden P, Wagers AJ et al (2013) M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16(9):1211–1218. https://doi.org/10.1038/nn.3469
Hagemeyer N, Hanft KM, Akriditou MA, Unger N, Park ES, Stanley ER, Staszewski O, Dimou L et al (2017) Microglia contribute to normal myelinogenesis and to oligodendrocyte progenitor maintenance during adulthood. Acta Neuropathol 134(3):441–458. https://doi.org/10.1007/s00401-017-1747-1
Bernhart E, Kollroser M, Rechberger G, Reicher H, Heinemann A, Schratl P, Hallstrom S, Wintersperger A et al (2010) Lysophosphatidic acid receptor activation affects the C13NJ microglia cell line proteome leading to alterations in glycolysis, motility, and cytoskeletal architecture. Proteomics 10(1):141–158. https://doi.org/10.1002/pmic.200900195
Barcia C, Ros CM, Annese V, Carrillo-De Sauvage MA, Ros-Bernal F, Gomez A, Yuste JE, Campuzano CM et al (2012) ROCK/Cdc42-mediated microglial motility and gliapse formation lead to phagocytosis of degenerating dopaminergic neurons in vivo. Sci Rep 2:809. https://doi.org/10.1038/srep00809
Paolicelli RC, Sierra A, Stevens B, Tremblay ME, Aguzzi A, Ajami B, Amit I, Audinat E et al (2022) Microglia states and nomenclature: a field at its crossroads. Neuron 110(21):3458–3483. https://doi.org/10.1016/j.neuron.2022.10.020
Funding
The authors acknowledge operating grant support from the Natural Science Foundation of Colleges and Universities in Anhui Province in 2020 (No. KJ2020A0976 and KJ2020A0144).
Author information
Authors and Affiliations
Contributions
Weizhuo Lu prepared figures and drafted the manuscript; Jiyue Wen edited and revised the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Informed Consent
Not applicable.
Competing Interests
The authors declare no competing interests.
Research Involving Human Participants and/or Animals
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, W., Wen, J. H2S-RhoA/ROCK Pathway and Glial Cells in Axonal Remyelination After Ischemic Stroke. Mol Neurobiol 60, 5493–5504 (2023). https://doi.org/10.1007/s12035-023-03422-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-023-03422-8