Abstract
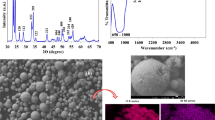
In this work, tertiary butylation of p-cresol was carried out in the presence of Cu1 − xCoxFe2O4 (x = 0 to 1) nanocatalysts by employing methyl-tert-butyl ether (MTBE) and tert-butyl alcohol (TBA) as alkylation agents. Effects of temperature, mole ratio, type and catalyst composition, time and solvent in reaction conditions were investigated. These nanocatalysts were synthesized using hydrothermal method. The characterization of these catalysts was investigated by means of X-ray diffraction (XRD), Scanning Electron Microscopy (SEM) and Fourier Transform Infrared (FT-IR). These nanocatalysts can be recovered and recycled. A good correlation was found between the activity, in terms of p-cresol conversion and various product selectivities for this reaction, and also the acid–base properties of the catalysts. Nano-sized Cu0.5Co0.5Fe2O4, in comparison to the other nanocatalysts discussed in this report is the most active nanocatalyst. The only product of this reaction is 2-t-butyl p-cresol with selectivity of 100% and p-cresol conversion is 70%. The possible mechanism for this reaction system was discussed based on the reaction results. The reaction mechanism proposed involves the interaction of phenoxide from phenol and the tert-butyl cation from isobutene on Cu1 − xCoxFe2O4.

Cu1 − xCoxFe2O4 (x = 0 to 1) nanocatalysts have been used for tertiary butylation of p-cresol. Different conditions and selectivity required for the formulation of p-tert-butyl-p-cresol (TBC) and 2,6-di-tert-butyl-p-cresol (DTBC) have been studied and optimized.
Similar content being viewed by others
References
Chhabra J S, Athar J, Agranal J P and Singh H 1993 Rubber Compos. Process. Appl. 20 305
Ajit S, Nilesh S and Shriniwas S 2004 Appl. Catal. A: Gen. 267 5
Karthik M, Vinu A and Tripathi K N 2005 Micropor. Mater. 70 207
Gonzalez V 1981 Rubber Chem. Technol. 54 134
Emil D and Vasile H 2003 J. Catal. 218 249
Fiege H 2000 Ullmann’s Encyclopedia of Industrial Chemistry; Federal Republic of Germany, Bayer A G, Leverkusen, A19, p. 324
Thomas M, Bollapragada S R and Chinnakonda S G 2004 J. Catal. 222 107
Rajgopal R, Vetrivel R and Rao B S 2000 Catal. Lett. 65 99
Rao B S, Sreekumar K and Jyothi T M 1998 Indian Patent 2707/98
Sreekumar K, Jyothi T M, Mathew T, Talawar M B, Sugunan S and Rao B S 2000 J. Mol. Catal. A 159 327
Mathew T, Shylesh S, Devassy M B and Vijayaraj M 2004 Appl. Catal. A: Gen. 273 35
Mathew T, Vijayaraj M, Pai S, Tope B B, Hegde G S, Rao S B and Gopinath S C 2004 J. Catal. 227 175
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
ALAMDARI, R.F., HOSSEINABADI, Z. & KHOUZANI, M.F. Synthesis, characterization and investigation of catalytic activity of Cu 1 − x Co x Fe 2 O 4 nanocatalysts in t-butylation of p-cresol. J Chem Sci 124, 827–834 (2012). https://doi.org/10.1007/s12039-012-0269-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-012-0269-6