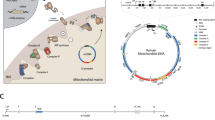
Abstract
The potential role of the mitochondrial genome has recently attracted interest because of its high mutation frequency in tumors. Different aspects of mtDNA make it relevant for cancer‘s biology, such as it encodes a limited but essential number of genes for OXPHOS biogenesis, it is particularly susceptible to mutations, and its copy number can vary. Moreover, most ROS in mitochondria are produced by the electron transport chain. These characteristics place the mtDNA in the center of multiple signaling pathways, known as mitochondrial retrograde signaling, which modifies numerous key processes in cancer. Cybrid studies support that mtDNA mutations are relevant and exert their effect through a modification of OXPHOS function and ROS production. However, there is still much controversy regarding the clinical relevance of mtDNA mutations. New studies should focus more on OXPHOS dysfunction associated with a specific mutational signature rather than the presence of mutations in the mtDNA.



Similar content being viewed by others
Abbreviations
- OXPHOS:
-
Oxidative phosphorylation
- ROS:
-
Reactive oxygen species
- mtDNA:
-
Mitochondrial DNA
References
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. Elsevier Inc.; 2011;144:646–74.
Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012/03/20 ed. Elsevier Inc.; 2012;148:1145–59.
Larman TC, DePalma SR, Hadjipanayis AG, Protopopov A, Zhang J, Gabriel SB, et al. Spectrum of somatic mitochondrial mutations in five cancers. Proc Natl Acad Sci USA. 2012;109:14087–91.
Ju YS, Alexandrov LB, Gerstung M, Martincorena I, Nik-Zainal S, Ramakrishna M, et al. Origins and functional consequences of somatic mitochondrial DNA mutations in human cancer. Elife. 2014;3:1–28.
Stewart JB, Alaei-Mahabadi B, Sabarinathan R, Samuelsson T, Gorodkin J, Gustafsson CM, et al. Simultaneous DNA and RNA mapping of somatic mitochondrial mutations across diverse human cancers. PLoS Genet. 2015;11:e1005333.
Anderson S, Bankier AT, Barrell BG, de Bruijn MH, Coulson AR, Drouin J, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–65.
Greaves LC, Reeve AK, Taylor RW, Turnbull DM. Mitochondrial DNA and disease. J Pathol. 2012;226:274–86.
Itsara LS, Kennedy SR, Fox EJ, Yu S, Hewitt JJ, Sanchez-Contreras M, et al. Oxidative stress is not a major contributor to somatic mitochondrial DNA mutations. PLoS Genet. 2014;10:e1003974.
Pinto M, Moraes CT. Mechanisms linking mtDNA damage and aging. Free Radic Biol Med Elsevier. 2015;85:250–8.
Liou C-W, Lin T-K, Chen J-B, Tiao M-M, Weng S-W, Chen S-D, et al. Association between a common mitochondrial DNA D-loop polycytosine variant and alteration of mitochondrial copy number in human peripheral blood cells. J Med Genet. 2010;47:723–8.
Campbell CT, Kolesar JE, Kaufman B a. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta. Elsevier B.V.; 2012;1819:921–9.
Guo J, Zheng L, Liu W, Wang X, Wang Z, Wang Z, et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 2011;71:2978–87.
van Osch FHM, Voets AM, Schouten LJ, Gottschalk RWH, Simons CCJM, van Engeland M, et al. Mitochondrial DNA copy number in colorectal cancer: between tissue comparisons, clinicopathological characteristics and survival. Carcinogenesis. 2015;36:1502–10.
Mi J, Tian G, Liu S, Li X, Ni T, Zhang L, et al. The relationship between altered mitochondrial DNA copy number and cancer risk: a meta-analysis. Sci. Rep. Nature Publishing Group; 2015;5:10039.
Lin C-S, Wang L-S, Tsai C-M, Wei Y-H. Low copy number and low oxidative damage of mitochondrial DNA are associated with tumor progression in lung cancer tissues after neoadjuvant chemotherapy. Interact CardioVasc Thorac Surg. 2008;7:954–8.
Reznik E, Miller ML, Şenbabaoğlu Y, Riaz N, Sarungbam J, Tickoo SK, et al. Mitochondrial DNA copy number variation across human cancers. Elife. 2016;5:1–20.
Pyle A, Hudson G, Wilson IJ, Coxhead J, Smertenko T, Herbert M, et al. Extreme-depth re-sequencing of mitochondrial DNA Finds no evidence of paternal transmission in humans. PLoS Genet. 2015;11:e1005040.
Ruiz-Pesini E, Mishmar D, Brandon M. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science (80-.). 2004;303:223–7.
Coskun P, Wyrembak J, Schriner SE, Chen H-W, Marciniack C, Laferla F, et al. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim. Biophys. Acta. Elsevier B.V.; 2012;1820:553–64.
Wang C, Wang Y, Wang H, Zhang R, Guo Z. Mitochondrial DNA haplogroup N is associated good outcome of gastric cancer. Tumour Biol. 2014;35:12555–9.
Kabekkodu SP, Bhat S, Mascarenhas R, Mallya S, Bhat M, Pandey D, et al. Mitochondrial DNA variation analysis in cervical cancer. Mitochondrion. © Elsevier B.V. and Mitochondria Research Society. All rights reserved.; 2014;16:73–82.
Wang Z, Choi S, Lee J, Huang Y-T, Chen F, Zhao Y, et al. Mitochondrial variations in non-small cell lung cancer (NSCLC) survival. Cancer Inf. 2015;14:1–9.
Weigl S, Paradiso A, Tommasi S. Mitochondria and familial predisposition to breast cancer. 2013;195–203.
Blein S, Bardel C, Danjean V, McGuffog L, Healey S, Barrowdale D, et al. An original phylogenetic approach identified mitochondrial haplogroup T1a1 as inversely associated with breast cancer risk in BRCA2 mutation carriers. Breast Cancer Res. 2015;17:61.
Bai R-K, Leal SM, Covarrubias D, Liu A, Wong L-JC. Mitochondrial genetic background modifies breast cancer risk. Cancer Res. 2007;67:4687–94.
Lam ET, Bracci PM, Holly E a, Chu C, Poon A, Wan E, et al. Mitochondrial DNA sequence variation and risk of pancreatic cancer. Cancer Res. 2012;72:686–95.
Fang H, Shen L, Chen T, He J, Ding Z, Wei J, et al. Cancer type-specific modulation of mitochondrial haplogroups in breast, colorectal and thyroid cancer. BMC Cancer. 2010;10:421.
Booker LM, Habermacher GM, Jessie BC, Sun QC, Baumann AK, Amin M, et al. North American White Mitochondrial Haplogroups in Prostate and Renal Cancer. J Urol. 2006;175:468–73.
Gómez-Durán A, Pacheu-Grau D, López-Gallardo E, Díez-Sánchez C, Montoya J, López-Pérez MJ, et al. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Hum Mol Genet. 2010;19:3343–53.
D’Aquila P, Rose G, Panno ML, Passarino G, Bellizzi D. SIRT3 gene expression: a link between inherited mitochondrial DNA variants and oxidative stress. Gene Elsevier B.V.; 2012;497:323–9.
Pello, Martin MA, Carelli V, Nijtmans LG, Achilli A, Pala M, et al. Mitochondrial DNA background modulates the assembly kinetics of OXPHOS complexes in a cellular model of mitochondrial disease. Hum Mol Genet. 2008;17:4001–11.
Kenney MC, Chwa M, Atilano SR, Falatoonzadeh P, Ramirez C, Malik D, et al. Molecular and bioenergetic differences between cells with African versus European inherited mitochondrial DNA haplogroups: implications for population susceptibility to diseases. Biochim. Biophys. Acta. Elsevier B.V.; 2014;1842:208–19.
Abbott J a, Francklyn CS, Robey-Bond SM. Transfer RNA and human disease. Front Genet. 2014;5:158.
Damas J, Samuels DC, Carneiro J, Amorim A, Pereira F. Mitochondrial DNA rearrangements in health and disease—a comprehensive study. Hum Mutat. 2014;35:1–14.
Datta S, Ray A, Roy R, Roy B. Association of DNA sequence variation in mitochondrial DNA polymerase with mitochondrial DNA synthesis and risk of oral cancer. Gene. Elsevier B.V.; 2016;575:650–4.
Popanda O, Seibold P, Nikolov I, Oakes CC, Burwinkel B, Hausmann S, et al. Germline variants of base excision repair genes and breast cancer: a polymorphism in DNA polymerase gamma modifies gene expression and breast cancer risk. Int J Cancer. 2013;132:55–62.
Ratanajaraya C, Nishiyama H, Takahashi M, Kawaguchi T, Saito R, Mikami Y, et al. A polymorphism of the POLG2 gene is genetically associated with the invasiveness of urinary bladder cancer in Japanese males. J Hum Genet. 2011;56:572–6.
Ding L, Liu Y. Borrowing Nuclear DNA Helicases to Protect Mitochondrial DNA. Int J Mol Sci. 2015;16:10870–87.
Gandhi VV, Samuels DC. Correlated tissue expression of genes of cytoplasmic and mitochondrial nucleotide metabolisms in normal tissues is disrupted in transformed tissues. Nucleosides, Nucleotides Nucleic Acids. 2012;31:112–29.
Singh KK, Ayyasamy V, Owens KM, Koul MS, Vujcic M. Mutations in mitochondrial DNA polymerase-γ promote breast tumorigenesis. J. Hum. Genet. Nature Publishing Group. 2009;54:516–24.
Chen P-L, Chen C-F, Chen Y, Guo XE, Huang C-K, Shew J-Y, et al. Mitochondrial genome instability resulting from SUV3 haploinsufficiency leads to tumorigenesis and shortened lifespan. Oncogene. 2013;32:1193–201.
Yadav N, Chandra D. Mitochondrial DNA mutations and breast tumorigenesis. Biochim. Biophys. Acta - Rev. Cancer. Elsevier B.V.; 2013;1836:336–44.
Yuan Y, Wang W, Li H, Yu Y, Tao J, Huang S, et al. Nonsense and Missense Mutation of Mitochondrial ND6 gene promotes cell migration and invasion in human lung adenocarcinoma. BMC Cancer. 2015;15:346.
Xu H, He W, Jiang H-G, Zhao H, Peng X-H, Wei Y-H, et al. Prognostic value of mitochondrial DNA content and G10398A polymorphism in non-small cell lung cancer. Oncol Rep. 2013;30:3006–12.
Hey-Mogensen M, Goncalves RLS, Orr AL, Brand MD. Production of superoxide/H2O2 by dihydroorotate dehydrogenase in rat skeletal muscle mitochondria. Free Radic Biol Med. 2014;72:149–55.
Fisher-Wellman KH, Gilliam LAA, Lin C-T, Cathey BL, Lark DS, Neufer PD. Mitochondrial glutathione depletion reveals a novel role for the pyruvate dehydrogenase complex as a key H2O2-emitting source under conditions of nutrient overload. Free Radic Biol Med. 2013;65:1201–8.
Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol Elsevier. 2015;4C:381–98.
Bleier L, Wittig I, Heide H, Steger M, Brandt U, Dröse S. Generator-specific targets of mitochondrial reactive oxygen species. Free Radic Biol Med Elsevier. 2015;78:1–10.
Peralta D, Bronowska AK, Morgan B, Dóka É, Van Laer K, Nagy P, et al. A proton relay enhances H2O2 sensitivity of GAPDH to facilitate metabolic adaptation. Nat Chem Biol 2015;11.
Desouki MM, Kulawiec M, Bansal S, Das GC, Singh KK. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol. Ther. Landes Bioscience Inc.; 2005;4:1367–73.
Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc Natl Acad Sci U S A. 2010/04/28 ed. 2010;107:8788–93.
Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2010/12/29 ed. 2011;21:103–15.
Sharma LK, Fang H, Liu J, Vartak R, Deng J, Bai Y. Mitochondrial respiratory complex I dysfunction promotes tumorigenesis through ROS alteration and AKT activation. Hum Mol Genet. 2011/09/06 ed. 2011;20:4605–16.
Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. Elsevier Inc.; 2010;40:294–309.
Porporato PE, Payen VL, Pérez-Escuredo J, De Saedeleer CJ, Danhier P, Copetti T, et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014;8:754–66.
Cruz-Bermúdez A, Vallejo C, Vicente-blanco RJ, Gallardo ME, Fernandez-Moreno MA, Quintanilla M, et al. Enhanced tumorigenicity by mitochondrial DNA mild mutations. Oncotarget. 2015;6:13628–43.
Formentini L, Sanchez-Arago M, Sanchez-Cenizo L, Cuezva JM. The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol Cell. 2012;45:731–42.
Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal. 2013/04/16 ed. 2013;19:1469–80.
Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordóñez Á, et al. Induction of the mitochondrial NDUFA4L2 protein by HIF-1α decreases oxygen consumption by inhibiting Complex I activity. Cell Metab. 2011;14:768–79.
Mailloux RJ, Harper M-E. Uncoupling proteins and the control of mitochondrial reactive oxygen species production. Free Radic. Biol. Med. Elsevier Inc.; 2011;51:1106–15.
Dröse S, Brandt U, Wittig I. Mitochondrial respiratory chain complexes as sources and targets of thiol-based redox-regulation. Biochim. Biophys. Acta. Elsevier B.V.; 2014;1844:1344–54.
Tuppen H a L, Blakely EL, Turnbull DM, Taylor RW. Mitochondrial DNA mutations and human disease. Biochim. Biophys. Acta. Elsevier B.V.; 2010;1797:113–28.
Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–4.
Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14:709–21.
King MP, Attardi G. Human cells lacking mtDNA: repopulation with exogenous mitochondria by complementation. Science (80-.). 1989/10/27 ed. 1989;246:500–3.
Yarham JW, Al-Dosary M, Blakely EL, Alston CL, Taylor RW, Elson JL, et al. A comparative analysis approach to determining the pathogenicity of mitochondrial tRNA mutations. Hum Mutat. 2011;32:1319–25.
Howell a N, Sager R. Tumorigenicity and its suppression in cybrids of mouse and Chinese hamster cell lines. Proc Natl Acad Sci USA. 1978;75:2358–62.
Hayashi J, Werbin H, Shay JW. Effects of normal human fibroblast mitochondrial DNA on segregation of HeLaTG Mitochondrial DNA and on tumorigenicity of HeLaTG cells. Cancer Res. 1986;46:4001–6.
Hayashi J, Takemitsu M, Nonaka I. Recovery of the missing tumorigenicity in mitochondrial DNA-less HeLa cells by introduction of mitochondrial DNA from normal human cells. Somat Cell Mol Genet. 1992;18:123–9.
Imanishi H, Hattori K, Wada R, Ishikawa K, Fukuda S, Takenaga K, et al. Mitochondrial DNA mutations regulate metastasis of human breast cancer cells. PLoS One. 2011;6:e23401.
Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci USA. 2005;102:719–24.
Shidara Y, Yamagata K, Kanamori T, Nakano K, Kwong JQ, Manfredi G, et al. Positive contribution of pathogenic mutations in the mitochondrial genome to the promotion of cancer by prevention from apoptosis. Cancer Res. 2005;65:1655–63.
Park JS, Sharma LK, Li H, Xiang R, Holstein D, Wu J, et al. A heteroplasmic, not homoplasmic, mitochondrial DNA mutation promotes tumorigenesis via alteration in reactive oxygen species generation and apoptosis. Hum Mol Genet. 2009;18:1578–89.
Kaipparettu BA, Ma Y, Wong LJ. Functional effects of cancer mitochondria on energy metabolism and tumorigenesis: utility of transmitochondrial cybrids. Ann N Y Acad Sci. 2010;1201:137–46.
Iommarini L, Kurelac I, Capristo M, Calvaruso MA, Giorgio V, Bergamini C, et al. Different mtDNA mutations modify tumor progression in dependence of the degree of respiratory complex I impairment. Hum Mol Genet. 2014;23:1453–66.
Calabrese C, Iommarini L, Kurelac I, Calvaruso MA, Capristo M, Lollini PL, et al. Respiratory complex I is essential to induce a Warburg profile in mitochondria-defective tumor cells. Cancer Metab. 2013;1:11.
Tan AS, Baty JW, Dong L-F, Bezawork-Geleta A, Endaya B, Goodwin J, et al. Mitochondrial Genome Acquisition Restores Respiratory Function and Tumorigenic Potential of Cancer Cells without Mitochondrial DNA. Cell Metab. Elsevier Inc.; 2015;21:81–94.
Berridge MV, Dong L, Neuzil J. Mitochondrial DNA in tumor initiation, progression, and metastasis: role of horizontal mtDNA transfer. Cancer Res. 2015;75:3203–8.
Wallace DC. Mitochondria and cancer. Nat. Rev. Cancer. Nature Publishing Group; 2012;12:685–98.
Horan MP, Gemmell NJ, Wolff JN. From evolutionary bystander to master manipulator: the emerging roles for the mitochondrial genome as a modulator of nuclear gene expression. Eur J Hum Genet. 2013;21:1335–7.
Ward PS, Thompson CB. metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. Elsevier. 2012;21:297–308.
Picard M, Zhang J, Hancock S, Derbeneva O, Golhar R, Golik P, et al. Progressive increase in mtDNA 3243A>G heteroplasmy causes abrupt transcriptional reprogramming. Proc Natl Acad Sci USA. 2014;111:E4033–42.
Amuthan G, Biswas G, Zhang SY, Klein-Szanto a, Vijayasarathy C, Avadhani NG. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 2001;20:1910–20.
Tang W, Chowdhury AR, Guha M, Huang L, Van Winkle T, Rustgi AK, et al. Silencing of IkBβ mRNA causes disruption of mitochondrial retrograde signaling and suppression of tumor growth in vivo. Carcinogenesis. 2012;33:1762–8.
Mitsushita J, Lambeth JD, Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res. 2004;64:3580–5.
Veatch JR, McMurray M a, Nelson ZW, Gottschling DE. Mitochondrial dysfunction leads to nuclear genome instability via an iron-sulfur cluster defect. Cell. Elsevier Ltd; 2009;137:1247–58.
Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–23.
Lee C, Zeng J, Drew BG, Sallam T, Martin-Montalvo A, Wan J, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21:443–54.
Yen K, Lee C, Mehta H, Cohen P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. 2013;50:R11–9.
Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003/05/07 ed. 2003;423:456–61.
Mottaghi-Dastjerdi N, Soltany-Rezaee-Rad M, Sepehrizadeh Z, Roshandel G, Ebrahimifard F, Setayesh N. Genome expression analysis by suppression subtractive hybridization identified overexpression of Humanin, a target gene in gastric cancer chemoresistance. Daru. 2014/01/10 ed. 2014;22:14.
Monaghan RM, Whitmarsh AJ. mitochondrial proteins moonlighting in the nucleus. Trends Biochem. Sci. Elsevier Ltd; 2015;xx:1–8.
Ye X-Q, Li Q, Wang G-H, Sun F-F, Huang G-J, Bian X-W, et al. Mitochondrial and energy metabolism-related properties as novel indicators of lung cancer stem cells. Int J Cancer. 2011;129:820–31.
Menendez JA, Alarcón T. Metabostemness: a new cancer hallmark. Front. Oncol. 2014;4:262.
Guha M, Srinivasan S, Ruthel G, Kashina AK, Carstens RP, Mendoza A, et al. Mitochondrial retrograde signaling induces epithelial-mesenchymal transition and generates breast cancer stem cells. Oncogene. Macmillan Publishers Limited; 2014;33:5238–50.
Yang M, Yan M, Zhang R, Li J, Luo Z. Side population cells isolated from human osteosarcoma are enriched with tumor-initiating cells. Cancer Sci. 2011;102:1774–81.
Martins-Neves SR, Lopes ÁO, do Carmo A, Paiva A a, Simões PC, Abrunhosa AJ, et al. Therapeutic implications of an enriched cancer stem-like cell population in a human osteosarcoma cell line. BMC Cancer. BioMed Central Ltd; 2012;12:139.
Heiden M Vander, Cantley L, Thompson C. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science (80-.). 2009;324:1029–34.
Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–23.
Ahn CS, Metallo CM. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab. 2015;3:1.
Fliss MS, Usadel H, Caballero OL, Wu L, Buta MR, Eleff SM, et al. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science (80-.). 2000;287:1997–9.
Takeuchi H, Fujimoto A, Hoon DSB. Detection of mitochondrial DNA alterations in plasma of malignant melanoma patients. Ann N Y Acad Sci. 2004;1022:50–4.
Okochi O, Hibi K, Uemura T, Inoue S, Takeda S, Kanek T, et al. Detection of mitochondrial DNA alterations in the serum of hepatocellular carcinoma patients. Clin Cancer Res. 2002;8:2875–8.
Hibi K, Nakayama H, Yamazaki T, Takase T, Taguchi M, Kasai Y, et al. Detection of mitochondrial DNA alterations in primary tumors and corresponding serum of colorectal cancer patients. Int J Cancer. 2001;94:429–31.
Yu M, Wan YF, Zou QH. Cell-free circulating mitochondrial DNA in the serum: a potential non-invasive biomarker for Ewing’s Sarcoma. Arch Med Res Elsevier Inc; 2012;43:389–94.
Duberow DP, Brait M, Hoque MO, Theodorescu D, Sidransky D, Dasgupta S, et al. High-performance detection of somatic D-loop mutation in urothelial cell carcinoma patients by polymorphism ratio sequencing. 2016;94:1015–1024.
Jerónimo C, Nomoto S, Caballero OL, Usadel H, Henrique R, Varzim G, et al. Mitochondrial mutations in early stage prostate cancer and bodily fluids. Oncogene. 2001;20:5195–8.
Wong LJC, Lueth M, Li XN, Lau CC, Vogel H. Detection of mitochondrial DNA mutations in the tumor and cerebrospinal fluid of medulloblastoma patients. Cancer Res. 2003;63:3866–71.
Zhu W, Qin W, Bradley P, Wessel A, Puckett CL, Sauter ER. Mitochondrial DNA mutations in breast cancer tissue and in matched nipple aspirate fluid. Carcinogenesis. 2005;26:145–52.
Acknowledgements
Work in the authors’ laboratories is supported by “Instituto de Salud Carlos III” [PI13/01806 and PIE14/0064 to M.P., PI10/0703 and PI13/00556 to R.G. and PI04/1001 to M.A.F.M.]; “Comunidad Autónoma de Madrid” [S2010/BMD-2402 to R.G.]; “Fundación Mutua Madrileña” [10.04.02.0064 to M.A.F.M.]. We thank Dr. Bruno Sainz Jr. for helpful suggestions to the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cruz-Bermúdez, A., Vicente-Blanco, R.J., Gonzalez-Vioque, E. et al. Spotlight on the relevance of mtDNA in cancer. Clin Transl Oncol 19, 409–418 (2017). https://doi.org/10.1007/s12094-016-1561-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-016-1561-6