Abstract
Purpose
To evaluate the efficacy and safety of oral weekly vinorelbine 60 mg/m2 for metastatic breast cancer (MBC) in patients previously treated with anthracyclines or taxanes in routine clinical practice.
Materials and methods
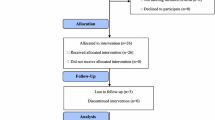
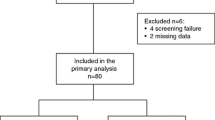
Fifty-five patients were enrolled in a prospective multicentre study conducted in Spain. Women ≥ 18 years of age with locally advanced breast cancer who were not candidates for surgical treatment with a radical intention or patients with stage IV disease, and who had received a prior taxane or anthracycline regimen were eligible for participation.
Results
Median age was 67 years. Median progression-free survival was 3.7 months (95% CI 2.5–4.9), median overall survival 10 months (95% CI 6.6–13.5), and overall response rate and clinical benefit rate were 29.1% and 49.1%, respectively. Main grade 3 and 4 toxicities were neutropenia 9.1%, febrile neutropenia 3.6% and constipation 3.6%. In total, 86% of the patients received complete treatment without delays or dose reduction. Moreover, HER2-positive patients who received oral vinorelbine concomitantly with trastuzumab showed better response (complete response: HER2-positive 14.3% vs. HER2-negative 0%; partial response: HER2-positive 42.9% vs. HER2-negative 25.6%; p = 0.008), better disease control rate (HER2-positive 100% vs. HER2-negative 46.2%; p = 0.011), and better values for the remaining analysed variables than HER2-negative patients.
Conclusion
Our study provides real-world data on the use of oral weekly vinorelbine, which proves an effective and well-tolerated regimen for MBC patients previously treated with taxanes or anthracyclines. Patients with HER2-positive disease could also benefit from this treatment in combination with trastuzumab.



Similar content being viewed by others
References
Rabow M, Small R, Jow A, Majure M, Chien A, Melisko M, et al. The value of embedding: integrated palliative care for patients with metastatic breast cancer. Breast Cancer Res Treat. 2018;167(3):703–8. https://doi.org/10.1007/s10549-017-4556-2.
Fedele P, Ciccarese M, Surico G, Cinieri S. An update on first line therapies for metastatic breast cancer. Expert Opin Pharmacother. 2018;19(3):243–52. https://doi.org/10.1080/14656566.2018.1425680.
Greenberg PA, Hortobagyi GN, Smith TL, Ziegler LD, Frye DK, Buzdar AU. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol. 1996;14(8):2197–205.
Cancer B. Fact sheet. 2016. http://www.moph.gov.lb/Campaigns/Materials/FactSheet.pdf. Accessed 07 May 2018.
Heinemann V, Di Gioia D, Vehling-Kaiser U, Harich HD, Heinrich B, Welt A, et al. A prospective multicenter phase II study of oral and i.v. vinorelbine plus trastuzumab as first-line therapy in HER2-overexpressing metastatic breast cancer. Ann Oncol. 2011;22(3):603–8. https://doi.org/10.1093/annonc/mdq409.
National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology V. 1. 2010. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf.
Palmieri C, Krell J, James CR, Harper-Wynne C, Misra V, Cleator S, et al. Rechallenging with anthracyclines and taxanes in metastatic breast cancer. Nat Rev Clin Oncol. 2010;7(10):561–74. https://doi.org/10.1038/nrclinonc.2010.122.
Corona SP, Sobhani N, Ianza A, Roviello G, Mustacchi G, Bortul M, et al. Advances in systemic therapy for metastatic breast cancer: future perspectives. Med Oncol (Northwood, London, England). 2017;34(7):119. https://doi.org/10.1007/s12032-017-0975-5.
Potier P. The synthesis of Navelbine prototype of a new series of vinblastine derivatives. Semin Oncol. 1989;16(2 Suppl 4):2–4.
Binet S, Chaineau E, Fellous A, Lataste H, Krikorian A, Couzinier JP, et al. Immunofluorescence study of the action of navelbine, vincristine and vinblastine on mitotic and axonal microtubules. Int J Cancer. 1990;46(2):262–6.
Fumoleau P, Delgado FM, Delozier T, Monnier A, Gil Delgado MA, Kerbrat P, et al. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 1993;11(7):1245–52.
Garcia-Conde J, Lluch A, Martin M, Casado A, Gervasio H, De Oliveira C, et al. Phase II trial of weekly IV vinorelbine in first-line advanced breast cancer chemotherapy. Ann Oncol. 1994;5(9):854–7.
Liu G, Franssen E, Fitch MI, Warner E. Patient preferences for oral versus intravenous palliative chemotherapy. J Clin Oncol. 1997;15(1):110–5.
Depierre A, Freyer G, Jassem J, Orfeuvre H, Ramlau R, Lemarie E, et al. Oral vinorelbine: feasibility and safety profile. Ann Oncol. 2001;12(12):1677–81.
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–16.
Aapro M, Finek J. Oral vinorelbine in metastatic breast cancer: a review of current clinical trial results. Cancer Treat Rev. 2012;38(2):120–6. https://doi.org/10.1016/j.ctrv.2011.05.005.
Freyer G, Delozier T, Lichinister M, Gedouin D, Bougnoux P, His P, et al. Phase II study of oral vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 2003;21(1):35–40. https://doi.org/10.1200/jco.2003.09.057.
Trillet-Lenoir V, Sommer H, Delozier T, Koralewski P, Ruiz Simon A, Lichinister M, et al. Oral vinorelbine in metastatic breast cancer: long term results of 2 phase II studies. Eur J Cancer. 2004;2(3):137 (Supplement).
Bartsch R, Wenzel C, Pluschnig U, Hussian D, Sevelda U, Locker GJ, et al. Oral vinorelbine alone or in combination with trastuzumab in advanced breast cancer: results from a pilot trial. Cancer Chemother Pharmacol. 2006;57(5):554–8. https://doi.org/10.1007/s00280-005-0092-6.
Baweja M, Suman VJ, Fitch TR, Mailliard JA, Bernath A, Rowland KM, et al. Phase II trial of oral vinorelbine for the treatment of metastatic breast cancer in patients > or = 65 years of age: an NCCTG study. Ann Oncol. 2006;17(4):623–9. https://doi.org/10.1093/annonc/mdj130.
Steger GG, Dominguez A, Dobrovolskaya N, Giotta F, Tubiana-Mathieu N, Pecherstorfer M, et al. Single-agent oral vinorelbine as first-line chemotherapy for endocrine-pretreated breast cancer with bone metastases and no visceral involvement: NORBREAST-228 Phase II Study. Clin Breast Cancer. 2018;18(1):e41–7. https://doi.org/10.1016/j.clbc.2017.05.012.
Cazzaniga ME, Cortesi L, Ferzi A, Scaltriti L, Cicchiello F, Ciccarese M, et al. Metronomic chemotherapy with oral vinorelbine (mVNR) and capecitabine (mCAPE) in advanced HER2-negative breast cancer patients: is it a way to optimize disease control? Final results of the VICTOR-2 study. Breast Cancer Res Treat. 2016;160(3):501–9. https://doi.org/10.1007/s10549-016-4009-3.
Bergen E, Berghoff AS, Rudas M, Dubsky P, De Vries C, Sattlberger C, et al. Taxanes plus trastuzumab compared to oral vinorelbine plus trastuzumab in HER2-overexpressing metastatic breast cancer. Breast care (Basel, Switzerland). 2014;9(5):344–8. https://doi.org/10.1159/000368330.
Chen TW, Yeh DC, Chao TY, Lin CH, Chow LW, Chang DY, et al. A Phase I/II study of the combination of lapatinib and oral vinorelbine in HER2-positive metastatic breast cancer. Jpn J Clin Oncol. 2018;48(3):242–7. https://doi.org/10.1093/jjco/hyx188.
Farhat F, Kattan JG, Ghosn M. Oral vinorelbine in combination with trastuzumab as a first-line therapy of metastatic or locally advanced HER2-positive breast cancer. Cancer Chemother Pharmacol. 2016;77(5):1069–77. https://doi.org/10.1007/s00280-016-3027-5.
Andersson M, Lidbrink E, Bjerre K, Wist E, Enevoldsen K, Jensen AB, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2-positive breast cancer: the HERNATA study. J Clin Oncol. 2011;29(3):264–71. https://doi.org/10.1200/JCO.2010.30.8213.
Mansour M, Mourad C. Phase II study of single agent oral vinorelbine as first-line treatment in patients with HER-2 negative metastatic breast cancer. Cancer Chemother Pharmacol. 2013;72(2):429–35. https://doi.org/10.1007/s00280-013-2216-8.
Jensen LH, Osterlind K, Rytter C. Randomized cross-over study of patient preference for oral or intravenous vinorelbine in combination with carboplatin in the treatment of advanced NSCLC. Lung Cancer. 2008;62(1):85–91. https://doi.org/10.1016/j.lungcan.2008.02.009.
Barni S, Freier B, Garau I, Mouysset JL, Sediva M, Zamagni C, et al. Burden of advanced breast cancer for patients and caregivers in Europe: comparison of two treatment forms of vinorelbine, oral and intravenous. Curr Med Res Opin. 2016;32(11):1807–12. https://doi.org/10.1080/03007995.2016.1211518.
Welt A, Meldgaard P, Martoni A, Hansen O, Gebbia V, Fischer von Weikersthal L, et al. Improving chemotherapy capacity by switching from IV to oral vinorelbine. Eur J Oncol Pharm. 2010;4(3):14–8.
Acknowledgements
This study was funded by Laboratoires Pierre Fabre. Medical writing support was provided by Dr. Almudena Fuster-Matanzo of Medical Statistics Consulting S.L. (Valencia). The authors would like to give special thanks to the patients and their families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors had no conflict of interest in this study.
Ethical approval
The protocol of this trial and informed consent were approved by the ethics committee of each participating hospital and met the ethical principles stated in the Declaration of Helsinki. This study was classified by the Spanish Agency of Medicines and Health Products (AEMPS).
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Blancas, I., Aguirre, E., Morales, S. et al. Real-world data on the efficacy and safety of weekly oral vinorelbine in breast cancer patients previously treated with anthracycline or taxane-based regimens. Clin Transl Oncol 21, 459–466 (2019). https://doi.org/10.1007/s12094-018-1946-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1946-9