Abstract
Objective
To evaluate the role of diffusion-weighted magnetic resonance imaging (DW-MR) in hepatoblastoma as compared to multiphase contrast enhanced computed tomography (CECT) scans for detection of satellite lesions.
Methods
In this prospective study on new cases of hepatoblastoma, multiphase CECT scans and DW-MR were performed before initiation of chemotherapy. Results of interpretation were compared for detection of satellite lesions. PRETEXT grouping and risk categorization was done according to SIOPEL based on CECT scan.
Results
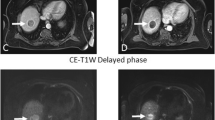
Nine boys and 1 girl with hepatoblastoma, with median age of 11.5 mo were included. All patients were stratified as high-risk group with 2 (20%) in PRETEXT II and 8 (80%) in PRETEXT III. In 2 of 10 (20%) patients, additional satellite lesions were detected on DW-MR, which upgraded their stage. One of the two patients had one satellite lesion identified on CECT, while additional seven satellite lesions were identified on DW-MR imaging. For the other patient, CECT showed no satellite lesion while DW-MR detected six satellite lesions.
Conclusion
MRI has become the gold standard investigation for evaluation of hepatoblastoma. DW-MR, which is a contrast free technique, is a better tool for assessment of satellite lesions which are usually missed on CECT. This would help in proper staging, planning of management and prognostication.



Similar content being viewed by others
References
Aronson DC, Meyers RL. Malignant tumors of the liver in children. Semin Pediatr Surg. 2016;25:265–75.
Czauderna P, Haeberle B, Hiyama E, et al. The children’s hepatic tumors international collaboration (CHIC): novel global rare tumor database yields new prognostic factors in hepatoblastoma and becomes a research model. Eur J Cancer. 2016;52:92–101.
Meyers RL, Aronson DC, Schweintiz DV, Zimmermann A, Malogolowkin MH. Pediatric Liver Tumor. In: Pizzo PA, Poplack DG, Adamson PC, Blaney SM, Helman LJ. Principles and practice of Pediatric Oncology, 7th ed. Philadelphia: Lippincott Williams and Wilkins; 2016. p. 838-860.
Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29:1725–48.
Hardie AD, Kizziah MK, Boulter DJ. Diagnostic accuracy of diffusion-weighted MRI for identifying hepatocellular carcinoma with liver explant correlation. J Med Imaging Radiat Oncol. 2011;55:362–7.
Roebuck DJ, Aronson D, Clapuyt P, et al ; International Childrhood Liver Tumor Strategy Group. 2005 PRETEXT: A revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr Radiol. 2007;37:123–32.
Das CJ, Dhingra S, Gupta AK, Iyer V, Agarwala S. Imaging of paediatric liver tumours with pathological correlation. Clin Radiol. 2009;64:1015–25.
Pugmire BS, Towbin AJ. Magnetic resonance imaging of primary pediatric liver tumors. Pediatr Radiol. 2016;46:764–77.
Meyers AB, Towbin AJ, Geller JI, Podberesky DJ. Hepatoblastoma imaging with gadoxetate disodium-enhanced MRI-typical, atypical, pre- and post-treatment evaluation. Pediatr Radiol. 2012;42:859–66.
McCarville MB, Roebuck DJ. Diagnosis and staging of hepatoblastoma: Imaging aspects. Pediatr Blood Cancer. 2012;59:793–9.
Koh DM, Collins DJ. Diffusion- weighted MRI in the body: applications and challenges in oncology. Am J Roentgenol. 2007;188:1622–35.
Gawande RS, Gonzalez G, Messing S, Khurana A, Daldrup-Link HE. Role of diffusion-weighted imaging in differentiating benign and malignant pediatric abdominal tumors. Pediatr Radiol. 2013;43:836–45.
Mitchell CL, Vasanawala SS. An approach to pediatric liver MRI. AJR Am J Roentgenol. 2011;196:W519–26.
Semeraro M, Branchereau S, Maibach R, et al. Relapses in hepatoblastoma patients: Clinical characteristics and outcome - experience of the International Childhood Liver Tumour Strategy Group (SIOPEL). Eur J Cancer. 2013;49:915–22.
Towbin AJ, Meyers RL, Woodley H, et al. 2017 PRETEXT: radiologic staging system for primary hepatic malignancies of childhood revised for the Paediatric Hepatic International Tumour Trial (PHITT). Pediatr Radiol. 2018;48:536–54.
Qiao GL, Li L, Cheng W, Ge J, Zhang Z, Wei Y. Predictors of survival after resection of children with hepatoblastoma: a single Asian center experience. Eur J Surg Oncol. 2014;40:1533–9.
Venkatramani R, Furman WL, Fuchs J, Warmann SW, Malogolowkin MH. Current and future management strategies for relapsed or progressive hepatoblastoma. Paediatr Drugs. 2012;14:221–32.
Fonseca A, Gupta A, Shaikh F, et al. Extreme hepatic resections for the treatment of advanced hepatoblastoma: are planned close margins an acceptable approach? Pediatr Blood Cancer. 2018;65: https://doi.org/10.1002/pbc.26820.
Otte JB, Pritchard J, Aronson DC, et al. Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer. 2004;42:74–83.
Tomizawa M, Shinozaki F, Fugo K, et al. Diagnostic accuracy of diffusion-weighted whole-body imaging with background body signal suppression/T2-weighted image fusion for the detection of abdominal solid cancer. Exp Ther Med. 2017;13:3509–15.
Acknowledgements
All the Residents of the department.
Author information
Authors and Affiliations
Contributions
SA, RS: Study conception and design; KS, AD, VJ, VB: Data acquisition; MJ, DK: Analysis and data interpretation; KS, SA: Drafting of the manuscript; DK, MJ, RS, AD, VJ, VB: Critical revision. SA will act as the guarantor for this paper.
Corresponding author
Ethics declarations
Consent to Participate
Written informed consent was obtained from the parent.
Consent for Publication
The patients signed informed consent regarding publishing their data and photographs.
Conflict of Interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sharma, K., Agarwala, S., Kandasamy, D. et al. Role of Diffusion Weighted MRI (DW-MR) in Detection of Satellite Lesions Not Detected with Multiphase CT Scans in Hepatoblastoma and Its Implications for Management. Indian J Pediatr 89, 968–974 (2022). https://doi.org/10.1007/s12098-021-04016-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12098-021-04016-9